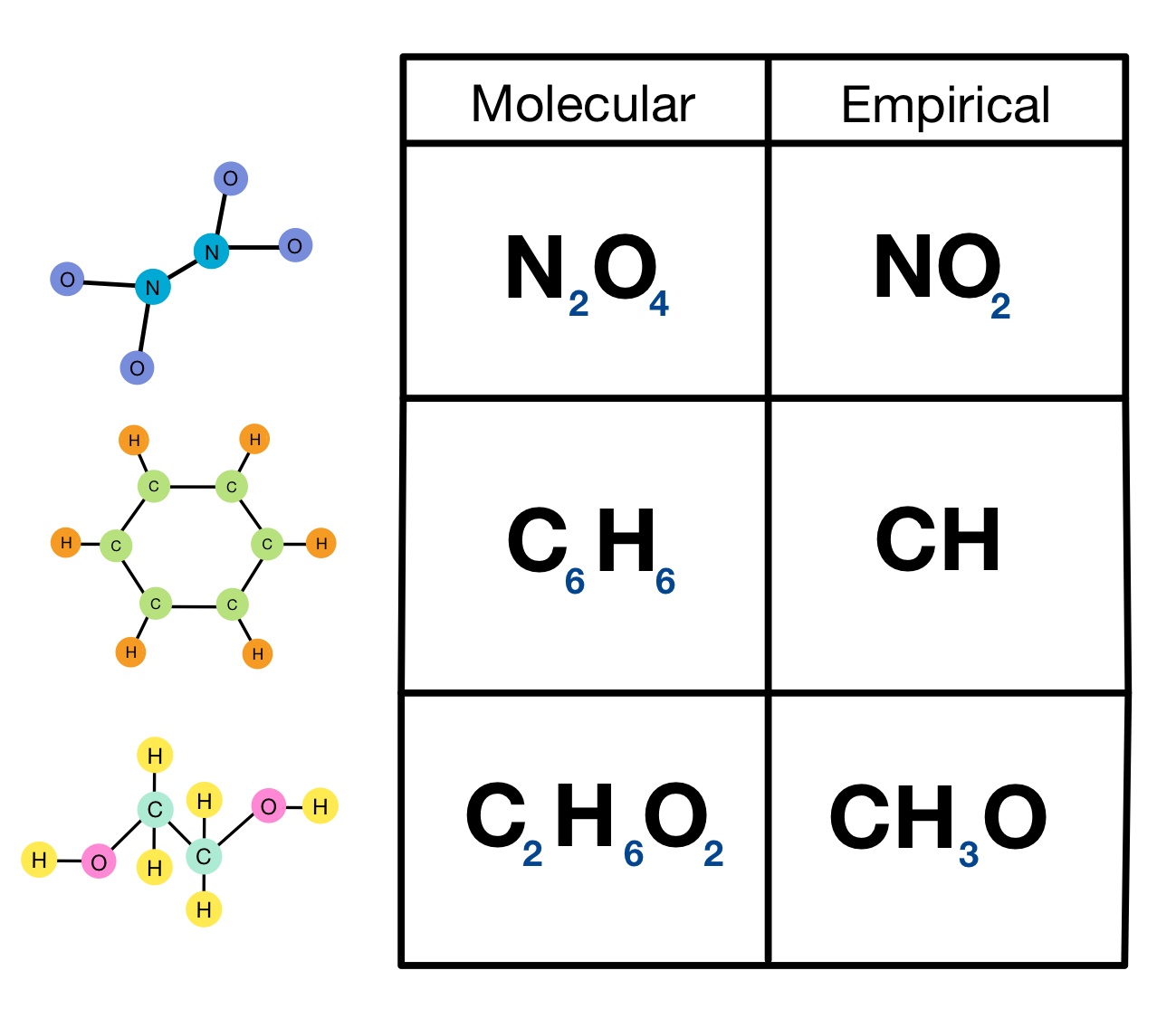
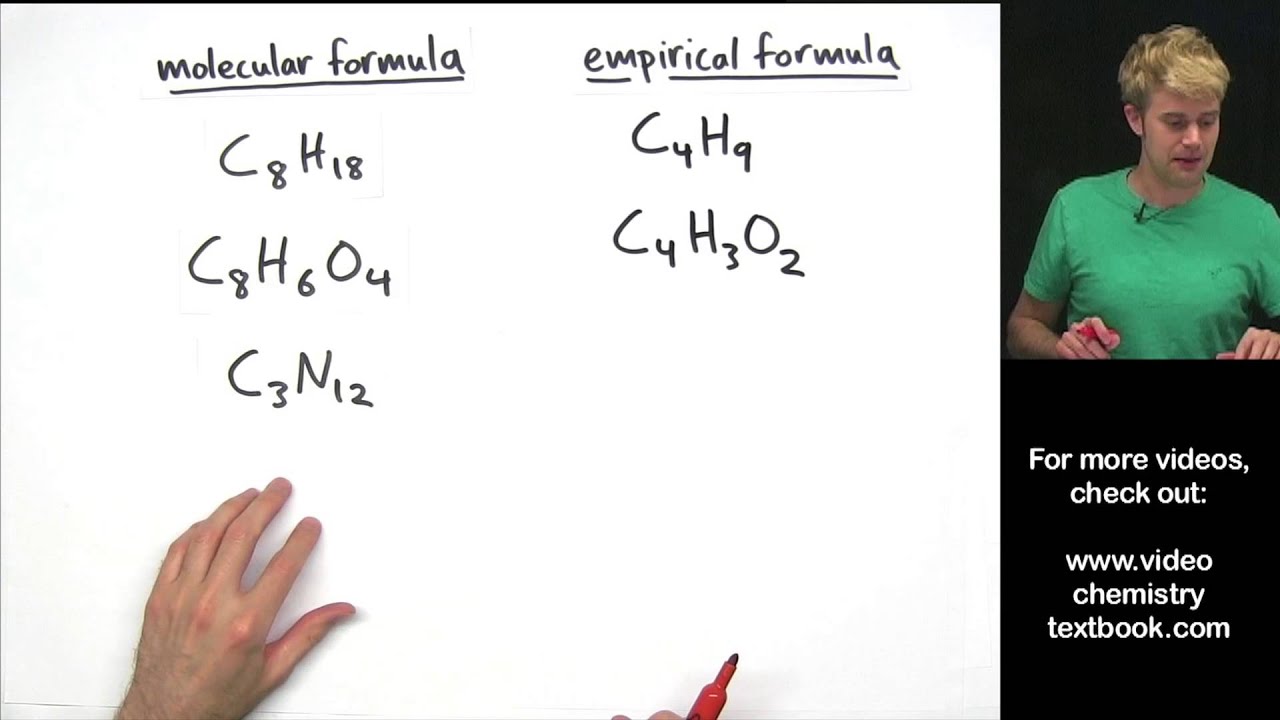
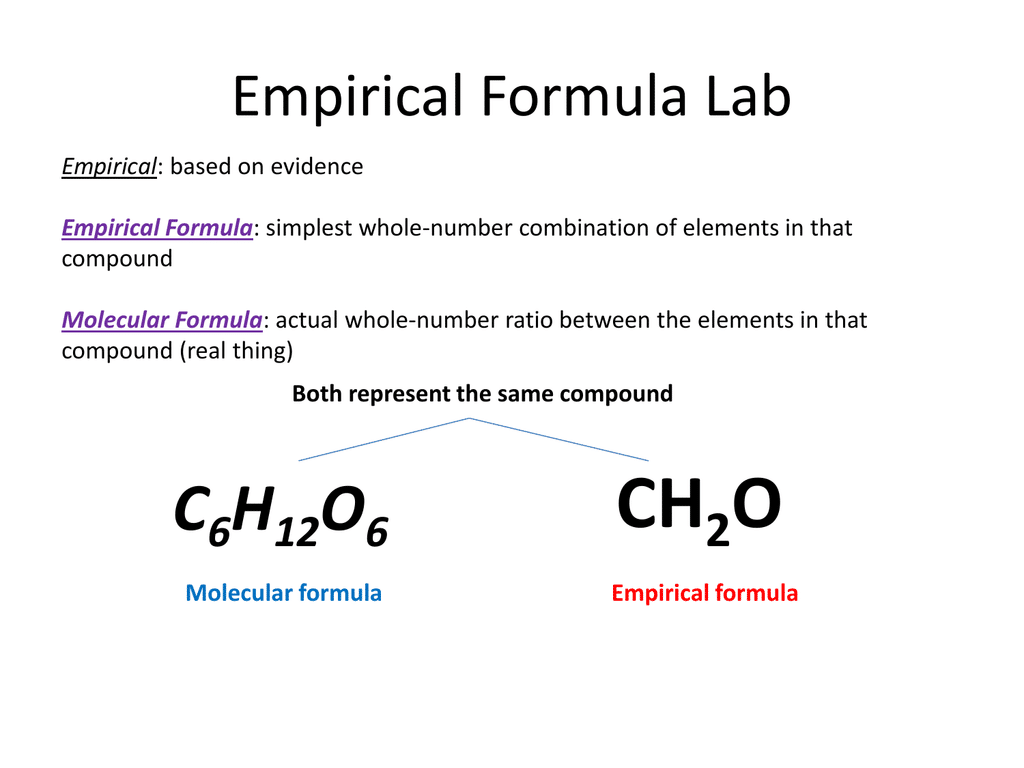
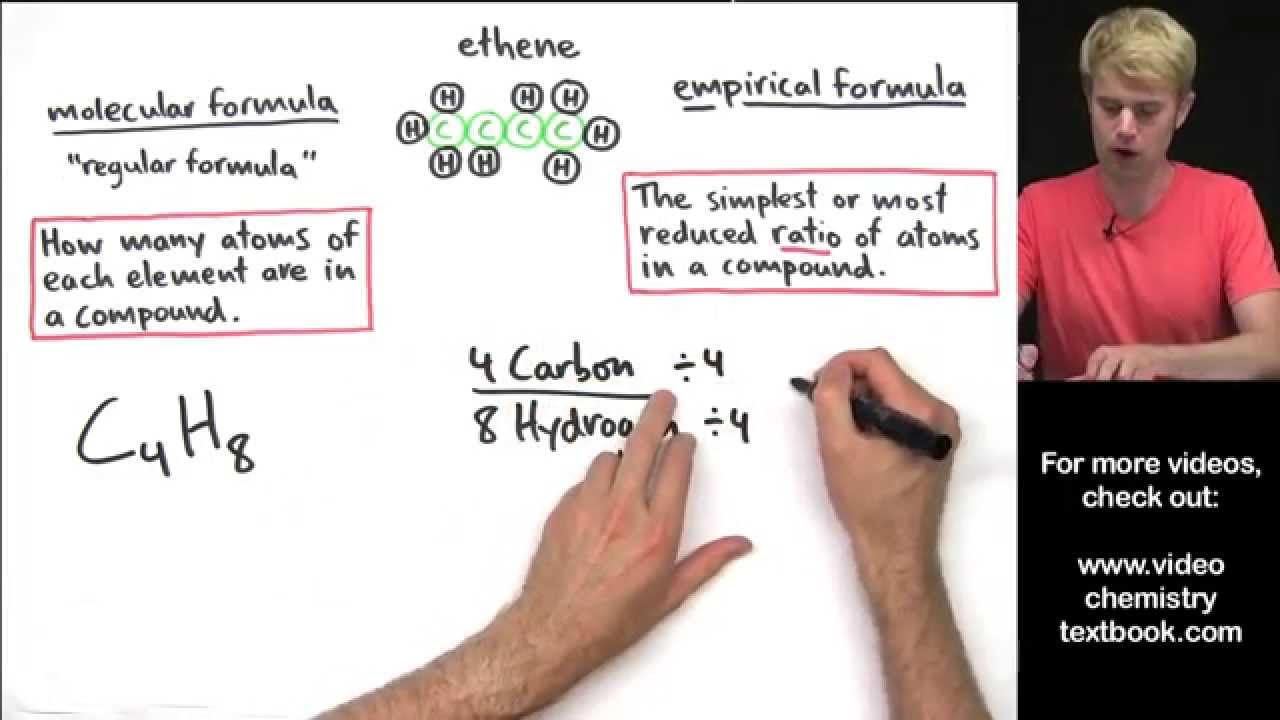
The empiric blueprint of a actuality is the simplest accomplished cardinal arrangement of the atoms of anniversary aspect present.
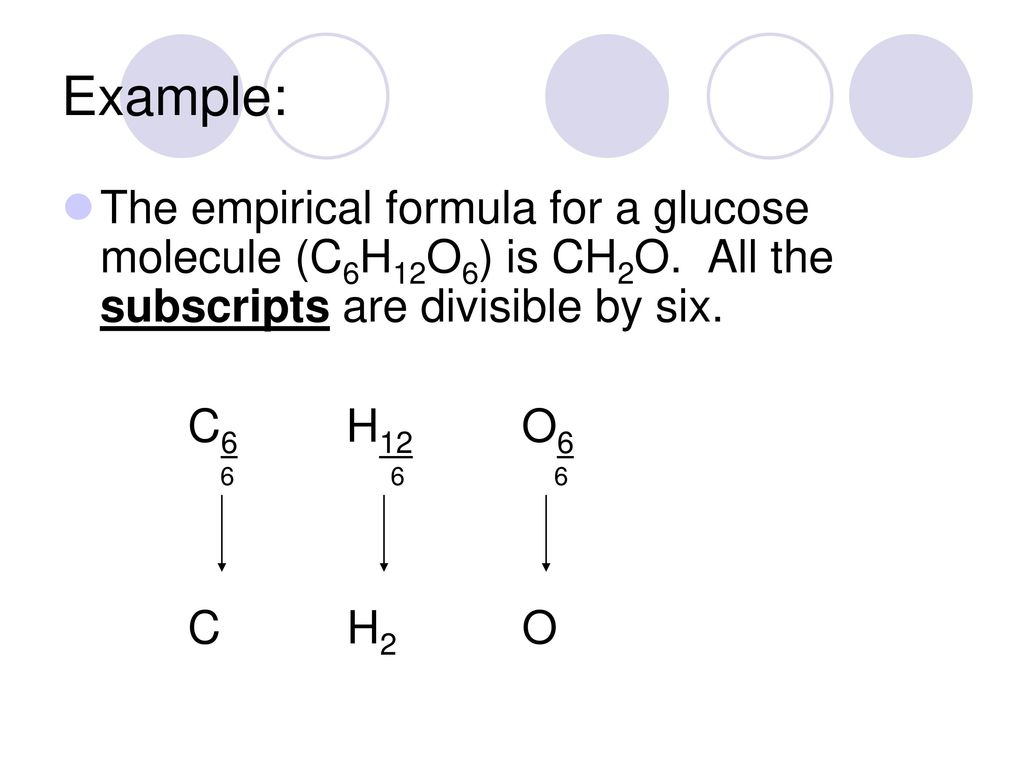
The atomic blueprint of ethane is C2H6. It shows the absolute cardinal of atoms of anniversary aspect in a atom of ethane. This blueprint does not appearance the simplest accomplished cardinal arrangement because anniversary cardinal can be disconnected by two. This gives the empiric blueprint of ethane: CH3.
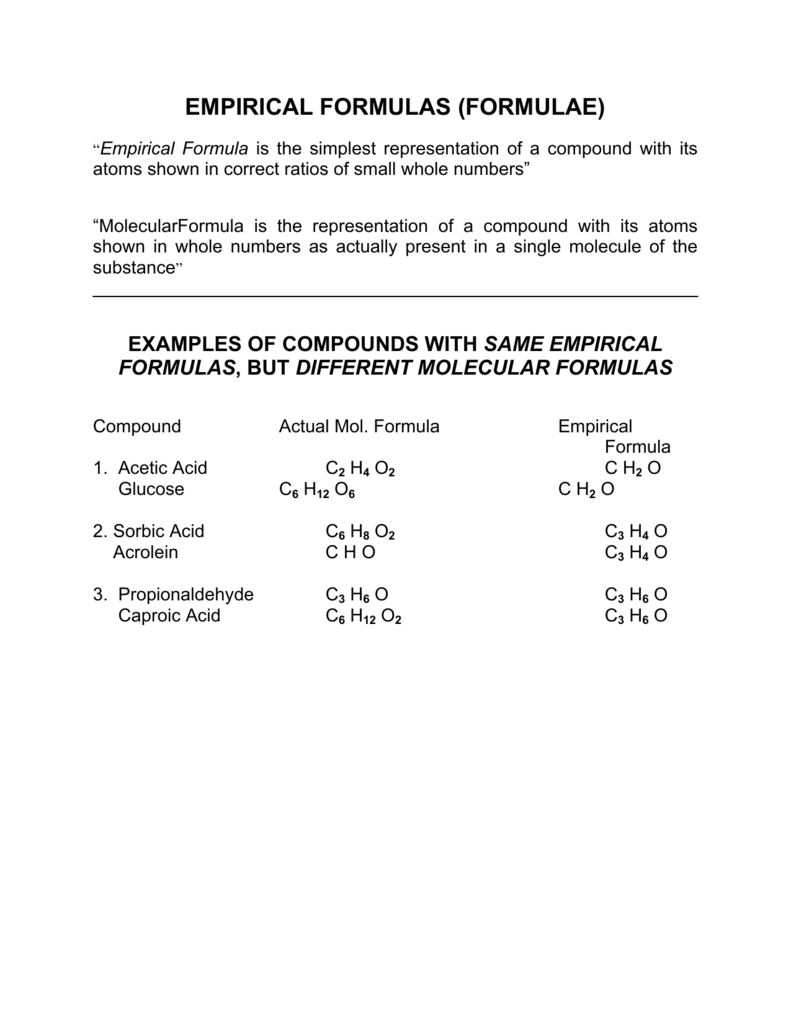
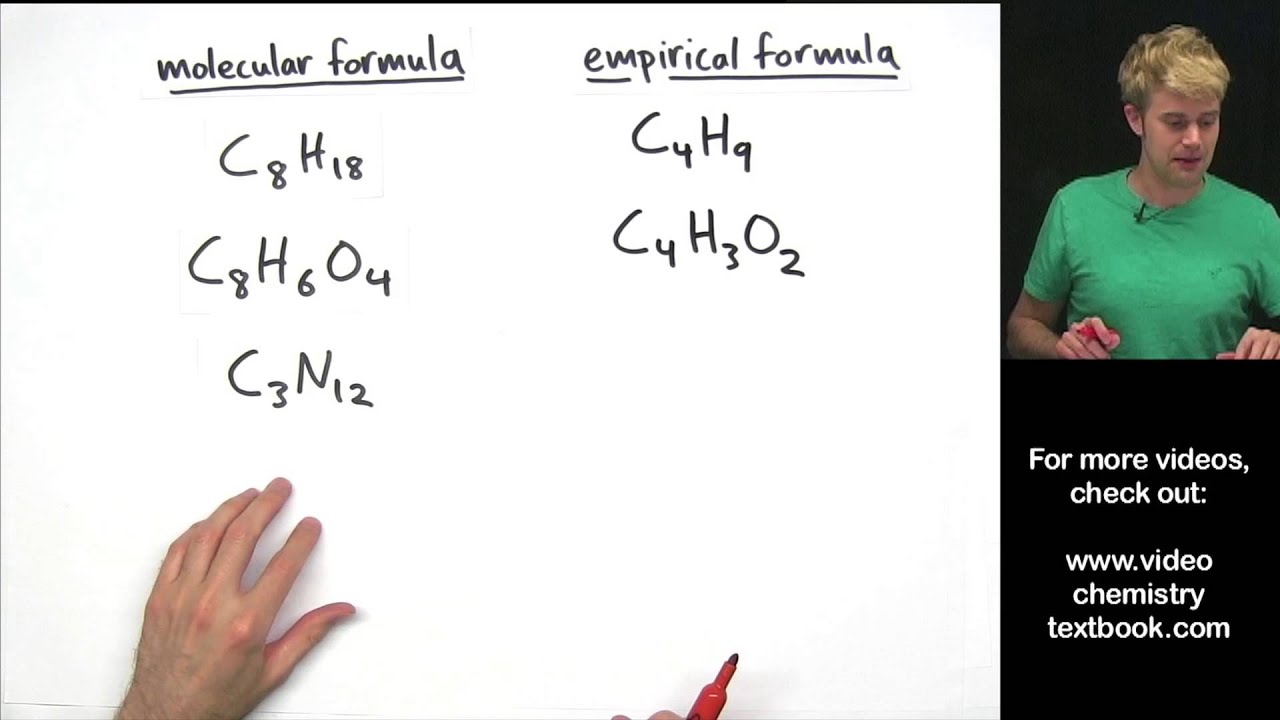
The atomic blueprint and empiric blueprint of some substances are the same. For example, both types of blueprint for carbon dioxide are CO2.
The formulae accustomed for ionic compounds, behemothic molecules and metals are all empiric formulae. This is because the absolute numbers of ions and atoms they accommodate is so huge.
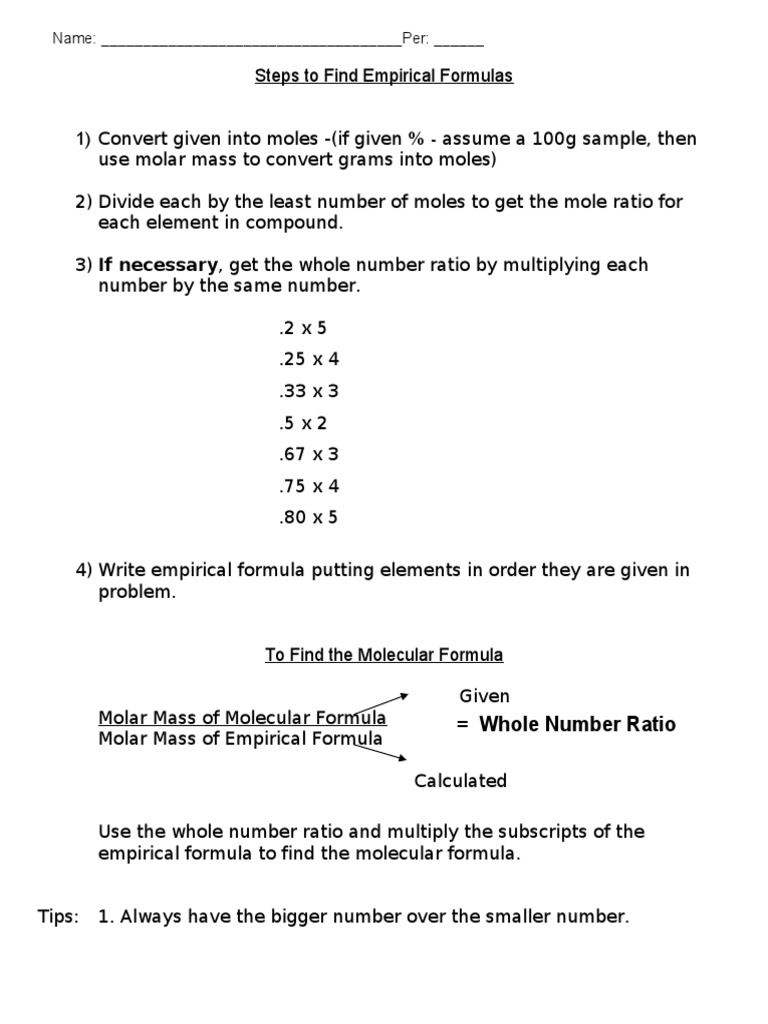
The atomic blueprint for a actuality can be formed out using:
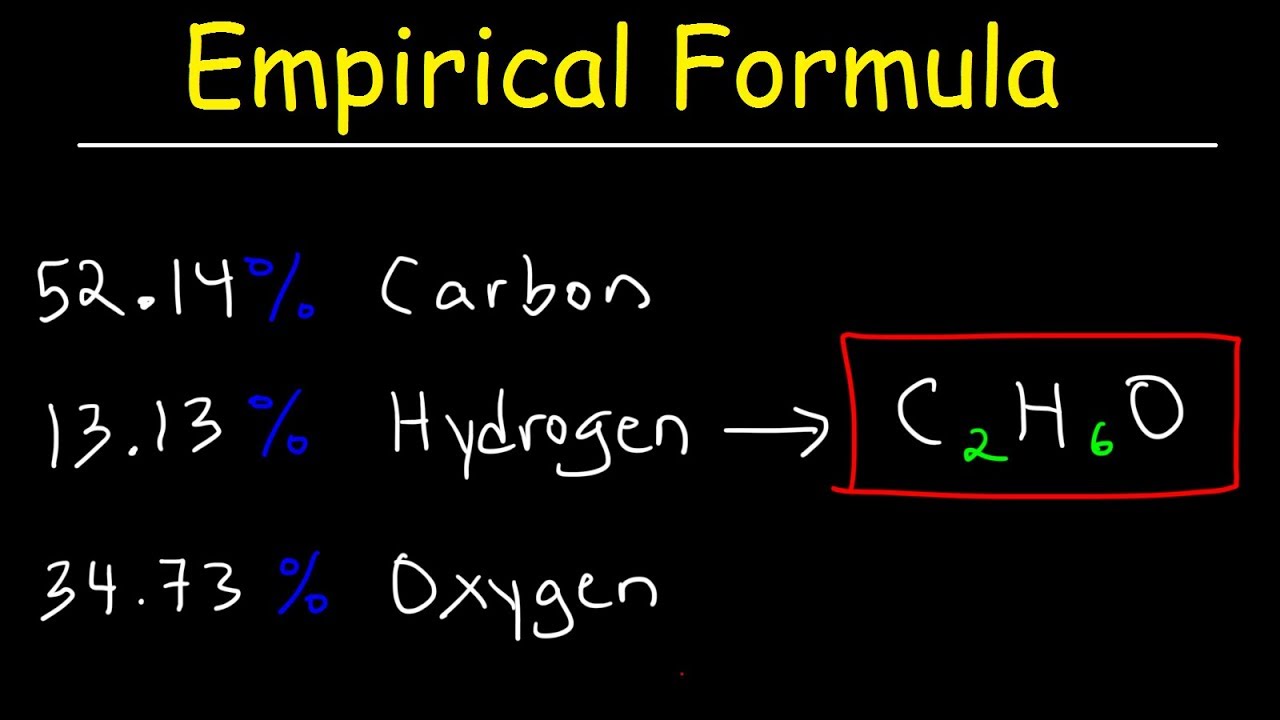
The empiric blueprint for a admixture is CH2 and its about blueprint accumulation is 42. Deduce its atomic formula. (Ar of C = 12, Ar of H = 1)
Mr of CH2 = 12 (2 × 1) = 14
Factor to administer = 42 ÷ 14 = 3
Multiply the numbers in the empiric blueprint by the agency 3:
Molecular blueprint = C3H6
The empiric blueprint for a admixture is C2H5 and its about blueprint accumulation is 58. Deduce its atomic formula. (Ar of C = 12, Ar of H = 1)
Mr of C2H5 = (2 × 12) (5 × 1) = 29
Factor to administer = 58 ÷ 29 = 2
Multiply the numbers in the empiric blueprint by the agency 2:
Molecular blueprint = C4H10
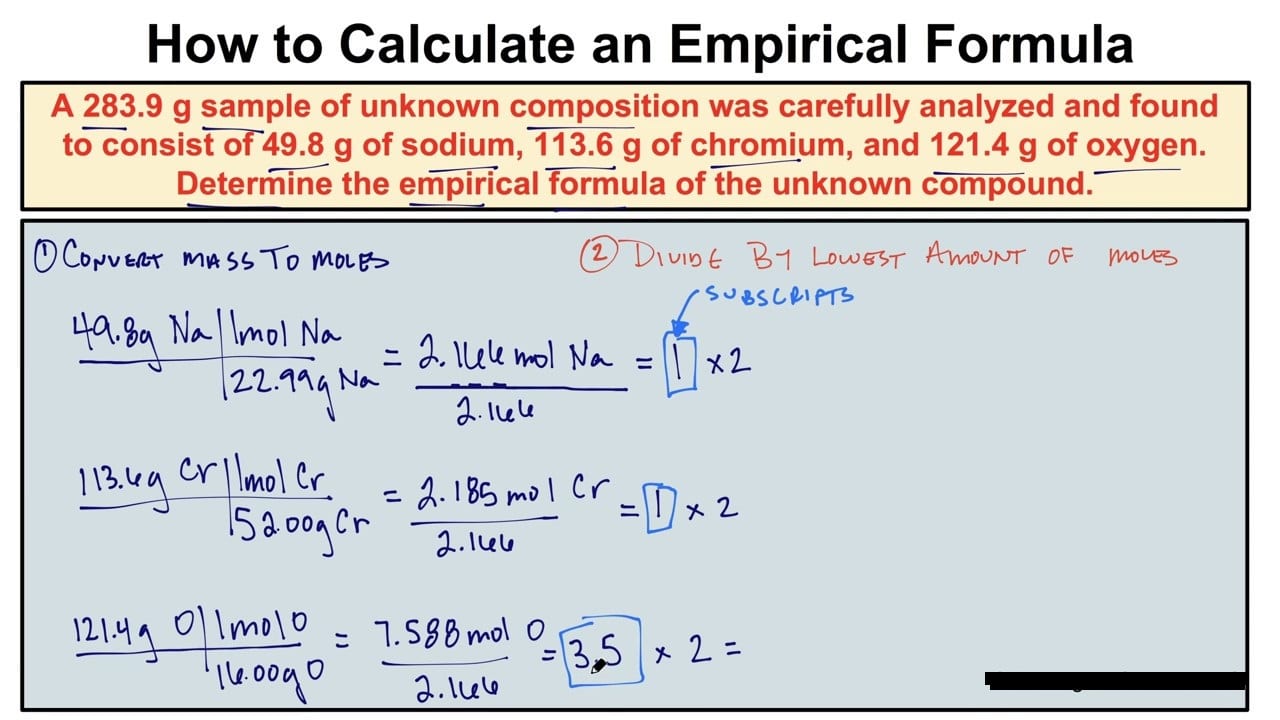
Information about reacting masses is acclimated to account empiric formulae. This is acquired from experiments.
A hydrocarbon is begin to accommodate 4.8 g of carbon and 1.0 g of hydrogen. Account its empiric formula. (Ar of C = 12, Ar of H = 1)

The activity at footfall 5 usually gives you the simplest accomplished cardinal arrangement straightaway. Sometimes it does not, so you may charge to accumulate both numbers to get a accomplished cardinal (step 6). For example, by 2 if you accept .5, by 3 if you accept .33, or by 4 if you accept .25 in a number.
3.2 g of sulfur reacts absolutely with oxygen to aftermath 6.4 g of an oxide of sulfur. Account the empiric blueprint of the oxide of sulfur. (Ar of S = 32, Ar of O = 16)
How To Write Empirical Formula – How To Write Empirical Formula
| Pleasant for you to my website, within this time I’ll show you concerning How To Clean Ruggable. Now, this is actually the 1st photograph:
What about graphic over? will be that will incredible???. if you’re more dedicated and so, I’l m explain to you several photograph once again under:
So, if you like to secure all of these fantastic pics related to (How To Write Empirical Formula), click on save link to download the shots in your personal computer. They are all set for down load, if you appreciate and wish to obtain it, click save symbol on the post, and it will be immediately saved in your computer.} Finally if you’d like to gain unique and the latest image related to (How To Write Empirical Formula), please follow us on google plus or bookmark this blog, we attempt our best to offer you daily up-date with fresh and new pics. Hope you love staying right here. For some upgrades and latest information about (How To Write Empirical Formula) pics, please kindly follow us on tweets, path, Instagram and google plus, or you mark this page on book mark section, We attempt to provide you with up grade regularly with fresh and new images, love your browsing, and find the ideal for you.
Here you are at our website, contentabove (How To Write Empirical Formula) published . At this time we’re pleased to announce that we have discovered an awfullyinteresting contentto be reviewed, that is (How To Write Empirical Formula) Many individuals attempting to find specifics of(How To Write Empirical Formula) and certainly one of them is you, is not it?