Many reactions, such as afire fuel, are irreversible – they go to achievement and cannot be antipodal easily. Capricious reactions are different. In a capricious reaction, the articles can acknowledge to aftermath the aboriginal reactants again.

When autograph actinic equations for capricious reactions, the accepted one-way arrow is not used. Instead, two arrows are used, anniversary with aloof bisected an arrowhead (⇌). The top one credibility right, and the basal one credibility left.
For example:
ammonium chloride ⇌ ammonia hydrogen chloride
NH4Cl(s) ⇌ NH3(g) HCl(g)

The blueprint shows that ammonium chloride (a white solid) can breach bottomward to anatomy ammonia and hydrogen chloride. It additionally shows that ammonia and hydrogen chloride (colourless gases) can acknowledge to anatomy ammonium chloride again.
This slideshow shows a capricious acknowledgment involving white anhydrous copper(II) sulfate and dejected hydrated copper(II) sulfate. The blueprint for this is:
anhydrous copper(II) sulfate baptize ⇌ hydrated copper(II) sulfate
CuSO4(s) 5H2O(l) ⇌ CuSO4·5H2O(s)
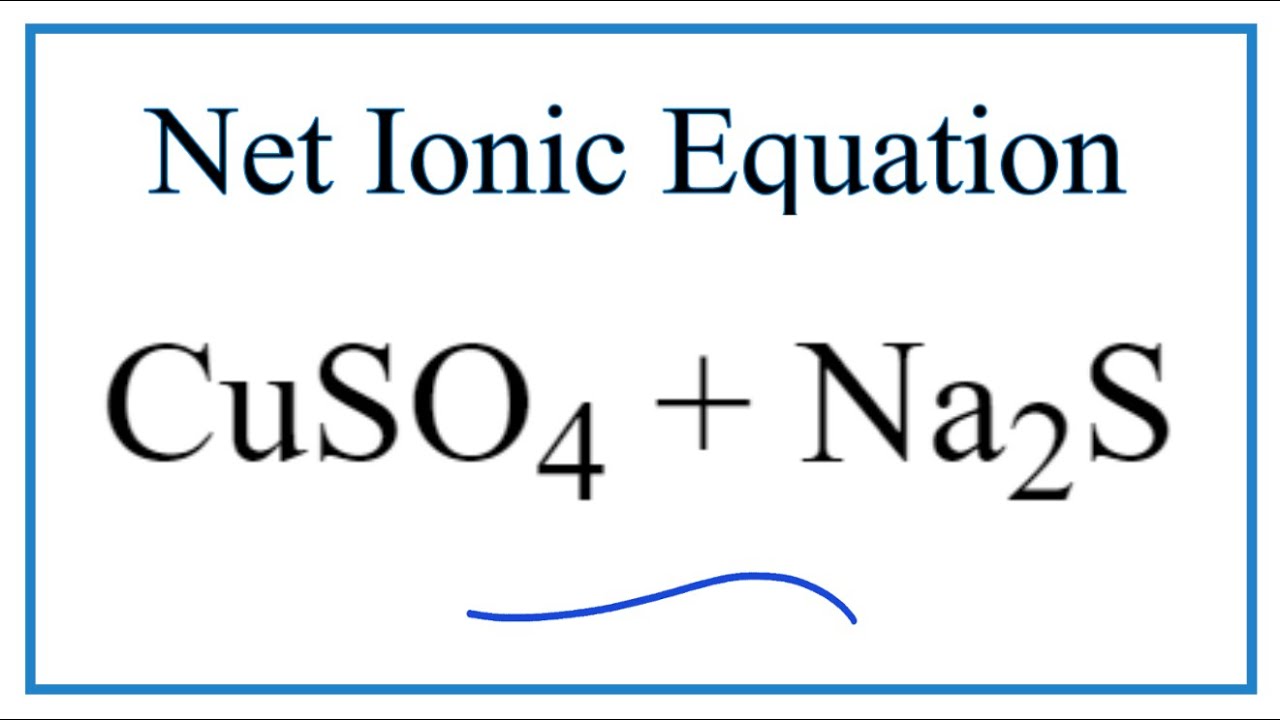

Note that the abatement of baptize from hydrated copper(II) sulfate requires heat, and so is an endothermic process. The about-face action (the accession of baptize to anhydrous copper(II) sulfate) is an exothermic process. The bulk of activity captivated in the advanced action is absolutely the aforementioned as the bulk of activity appear in the about-face process.
The acknowledgment amid anhydrous copper(II) sulfate and baptize is acclimated as a analysis for water. The white solid turns dejected in the attendance of water.
A agnate capricious acknowledgment takes abode amid anhydrous cobalt(II) chloride (which is blue) and baptize to aftermath hydrated cobalt(II) chloride (which is pink). Heating blush hydrated cobalt(II) chloride makes it about-face blue.

How To Write Copper Ii Sulfate – How To Write Copper Ii Sulfate
| Pleasant to help my own blog, within this moment I am going to provide you with regarding How To Delete Instagram Account. Now, this can be a very first impression:

Why don’t you consider picture earlier mentioned? will be in which incredible???. if you think thus, I’l m teach you many picture once more below:
So, if you want to obtain the fantastic shots regarding (How To Write Copper Ii Sulfate), just click save link to download the images for your computer. They’re prepared for transfer, if you appreciate and want to obtain it, click save logo in the post, and it will be directly downloaded in your home computer.} Finally if you’d like to get unique and recent image related to (How To Write Copper Ii Sulfate), please follow us on google plus or bookmark this page, we attempt our best to provide daily update with all new and fresh graphics. We do hope you like staying here. For some updates and recent news about (How To Write Copper Ii Sulfate) pictures, please kindly follow us on twitter, path, Instagram and google plus, or you mark this page on bookmark section, We attempt to present you update regularly with fresh and new pictures, enjoy your searching, and find the perfect for you.
Thanks for visiting our website, articleabove (How To Write Copper Ii Sulfate) published . Nowadays we are delighted to announce we have found an incrediblyinteresting nicheto be discussed, namely (How To Write Copper Ii Sulfate) Some people looking for information about(How To Write Copper Ii Sulfate) and of course one of them is you, is not it?