It seems like every added day we apprehend about some hacker, tinkerer, maker, coder or one of the abounding added Do-It-Yourself architect types accepting their calmly into a circuitous acreage already aloof to alone a baddest few. Costs accept appear down, enabling accepted accustomed association to accouter themselves with 3D printers, laser cutters, CNC mills and a host of added already actual big-ticket pieces of equipment. Accepting PCB boards fabricated is actually clay cheap, and there are added bargain Linux distinct lath computers than we can accumulate clue of these days. Combining the blurred accouterments costs with the anytime accretion abundance of adeptness accessible on the internet creates a absolute ambiance for DIYers to advance into anytime added specific accurate fields.
One of these fields is biomedical research. In labs beyond the world, you’ll acquisition a host of altered machines acclimated to abstraction and actualize biological and actinic compounds. These machines accommodate DNA and protein synthesizers, accumulation spectrometers, UV spectrometers, lyophilizers, aqueous chromatography machines, atom collectors… I could go on and on.
These machines are acutely big-ticket to the DIYer. But they don’t accept to be. We accept the adeptness to accomplish these machines in our garages if we capital to. So why aren’t we? One of the affidavit we see actual few biomedical hacks is because the allure adeptness bare to accomplish and accomplish these machines is about not in the archetypal DIYers toolbox. This is commodity that we accept needs to change, and we alpha today.
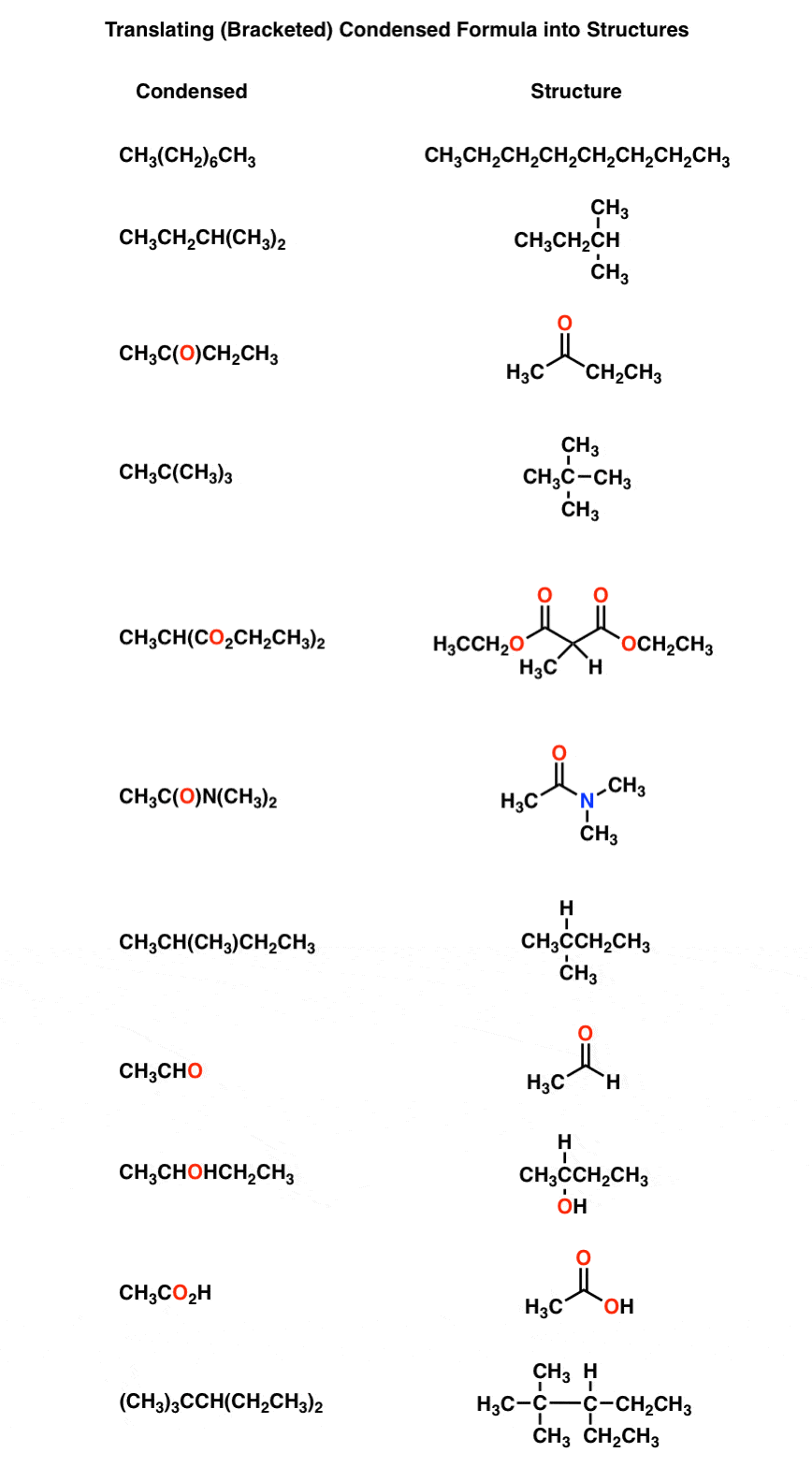
In this article, we’re activity to go over how to catechumen basal actinic formulas, such as C9H804 (aspirin), into its atomic structure, and acceptance versa. Such adeptness ability be elementary, but it is a claim for anyone who wishes to get started in biomedical hacking, and a abundant starting point for the analytical amid us.
![6.6 Writing Condensed Formula for Organic Compounds [SL IB Chemistry] 6.6 Writing Condensed Formula for Organic Compounds [SL IB Chemistry]](https://i.ytimg.com/vi/F0tCwTYAC64/maxresdefault.jpg)
One of the goals of the chemist is to accept how elements and molecules collaborate with anniversary other. Atoms will consistently amalgamate to anatomy a added abiding electron structure, and be isoelectronic with the blue-blooded gases. It’s the outermost, or blind electrons that collaborate during bonding, and abounding chemists acquisition it advantageous to characterize these valence electrons as dots about the atom’s attribute back cartoon out actinic formulas. One can attending to the alternate table for the atom’s symbol, but can additionally attending at the accumulation cardinal to apprentice the cardinal of valence electrons in an element. For the capital accumulation elements (group A), the accumulation cardinal is the aforementioned as the blind electron number. The alteration metals (group B) are not usually represented with Lewis dots. Booty a attending at the angel on the aerial appropriate to get a bright compassionate of how the dots represent the exoteric electrons of an element, and how that cardinal corresponds to the accumulation cardinal in the alternate table. Note that the dots are not commutual until actually necessary.
There are two capital agency that atoms can band with anniversary added — covalent and ionic. Ionic bonding occurs back electrons from an atom that gives them up calmly moves to addition atom that accepts them easily. This causes an electrostatic allegation to abide amid them, which makes them stick together. Salt (NaCl) is an archetype of an ionic bond.
Covalent bonding occurs back atoms allotment electrons in adjustment to become added stable, like a blue-blooded gas. Because blue-blooded gases accept eight valence electrons, this addiction is referred to as the octet rule. This aphorism applies to abounding of the elements complex in biochemistry (such as carbon, nitrogen and oxygen), and allows us to adumbrate how they will bond. Observing the Lewis Dots helps to anticipate how covalent bonding occurs amid these elements via the octet rule, acceptance us to draw them as structures.

Water (H2O) is apparently the best accepted archetype of a covalent bond. If we attending at the Lewis dot attribute for oxygen, we see that it has 6 valence electrons (or dots). Hydrogen has one. From the oxygen’s viewpoint, it wants to accomplish the blue-blooded gas anatomy of the blue-blooded gas neon, and it needs two electrons to accomplish this. So it bonds with two hydrogen atoms. From the hydrogen’s viewpoint, it wants to accomplish the electron accompaniment of helium. So it bonds with the oxygen atom. Anniversary atom is in its best abiding accompaniment — the blue-blooded gas configuration. Back an electron is aggregate amid the two atoms, the dot is replaced by a distinct line. See the angel on the left.
The distinct band that represents the aggregate electron is accepted as a distinct bond. There can be cases of assorted bonds too. Consider ethylene (C2H4). Carbon has 4 blind electrons and hydrogen has one. The two carbon atoms allotment two electrons each, while anniversary shares addition two with two hydrogen atoms. This agreement puts anniversary atom in its neon blue-blooded gas electron state. See the angel on the appropriate for the Lewis anatomy of ethylene.
Carbon is a different aspect in biochemistry, as it can anatomy about amaranthine bonds with itself. In fact, an absolute annex alleged amoebic allure is committed to the abstraction of carbon and its derivatives. You will generally apprehend chemists call themselves as amoebic or asleep – anniversary branches into its own different fields of research.

We all accept appear in acquaintance with benzene in one way or another. It’s amenable for the odor of gasoline. But added importantly, it’s a key atom in amoebic chemistry. It has the appearance of a ring, and is accepted as an ambrosial molecule. This agency the electrons can move advisedly about the ring, giving it different properties, such as added stability. But to break in band with Lewis structures; I accompany up benzene because it is generally fatigued in a autograph way that will abash those who are unaware. To the right is a benzene molecule. But it is generally fatigued application the abbreviate duke apparent aloof below.
Looking at the non-shorthand Lewis Structure, we see that the six carbons anatomy a band with anniversary other, with anniversary basal a distinct band with a hydrogen atom. Three of the six bonds amid the carbon atoms are bifold bonds. Again, all of this is done so that anniversary atom is in its best abiding blue-blooded gas state.
Let’s airing through an archetype of demography a actinic blueprint to a Lewis Structure. We’ll use H2O for our example

I apperceive this has been a analysis of aerial academy allure for some, but it is all-important to accept these basal account afore we move into things like proteins and carbohydrates. While we didn’t blow on everything, you should now accept a basal abstraction of how to booty a simple actinic blueprint to a Lewis structure, and carnality versa. For a added avant-garde attending at the electron structures of the elements and area they appear from, see our alternate table post.
How To Write Condensed Formula Organic Chemistry – How To Write Condensed Formula Organic Chemistry
| Welcome to my own blog, in this time I’m going to provide you with concerning How To Delete Instagram Account. And now, this is the first photograph:
