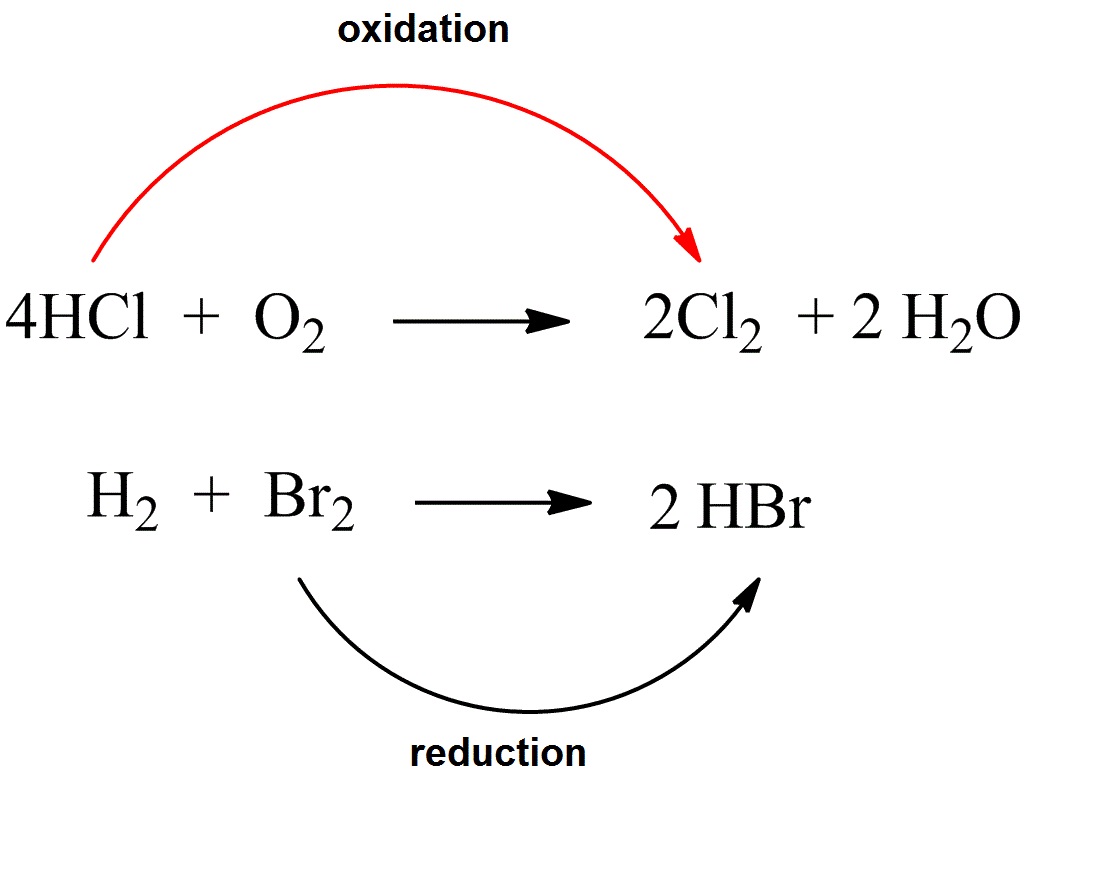
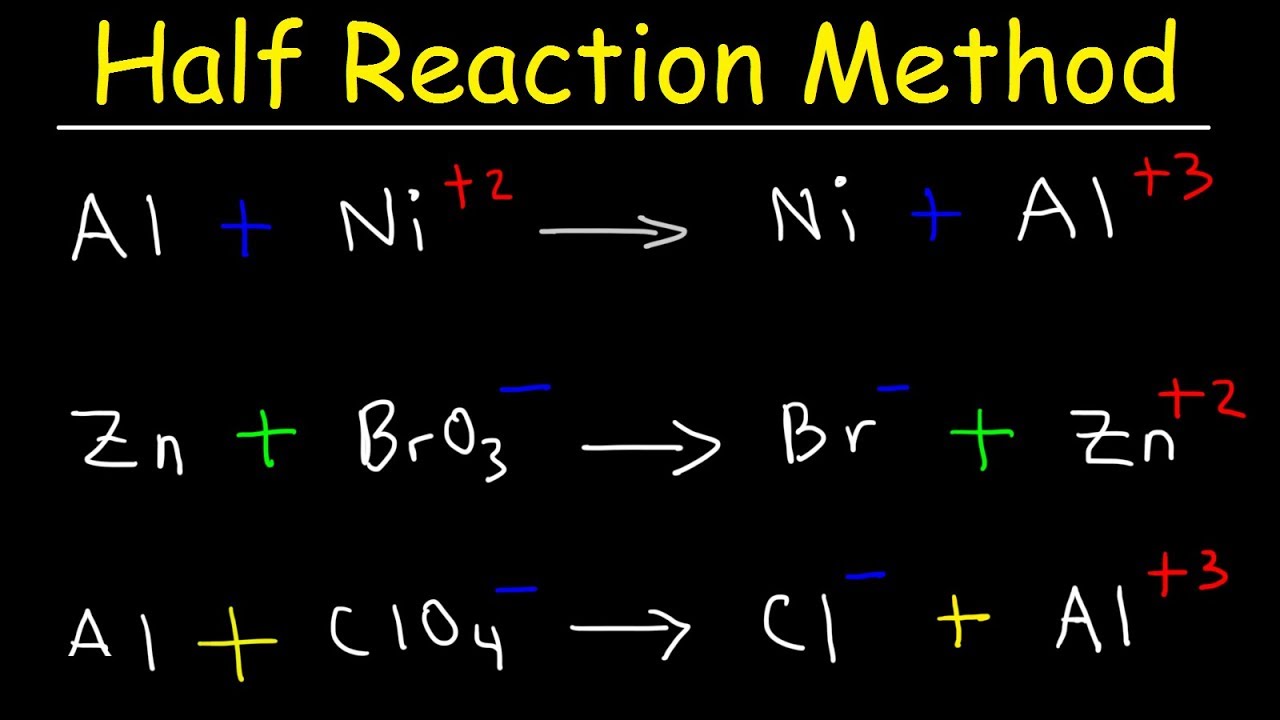
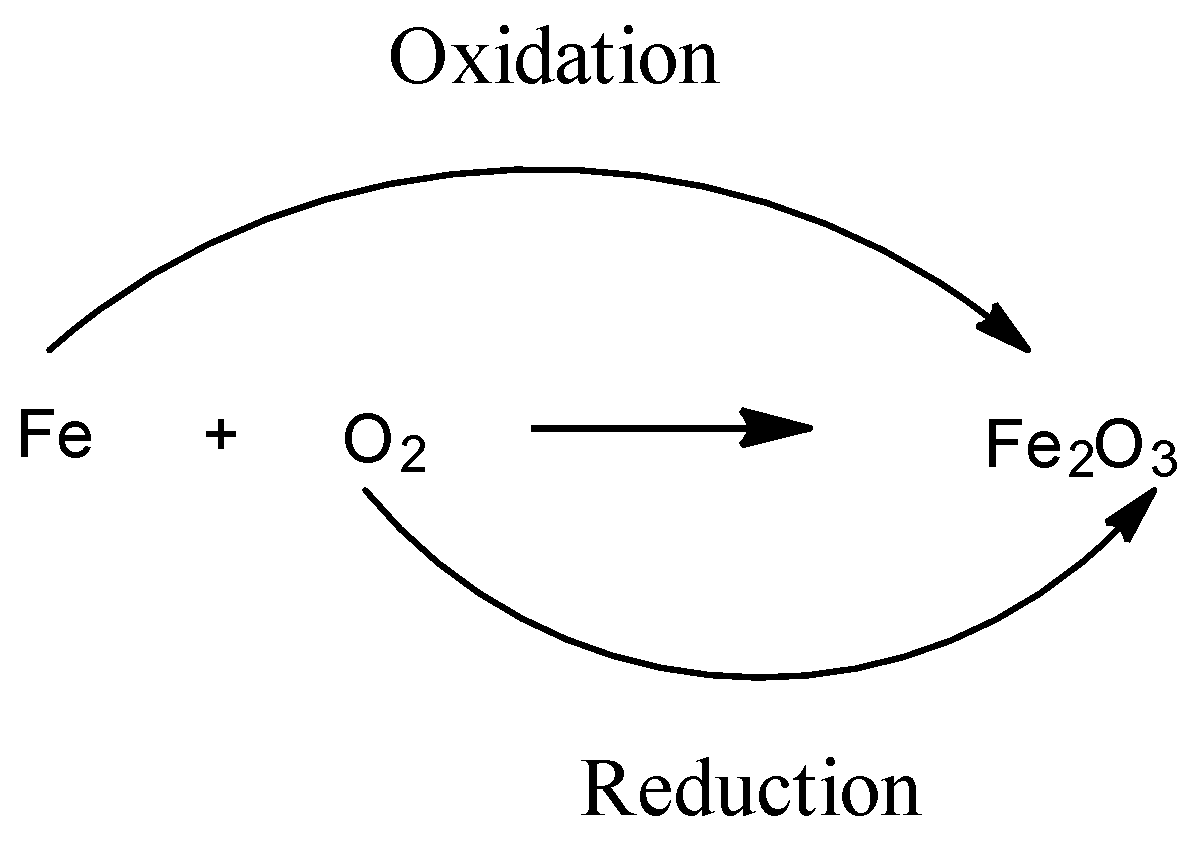
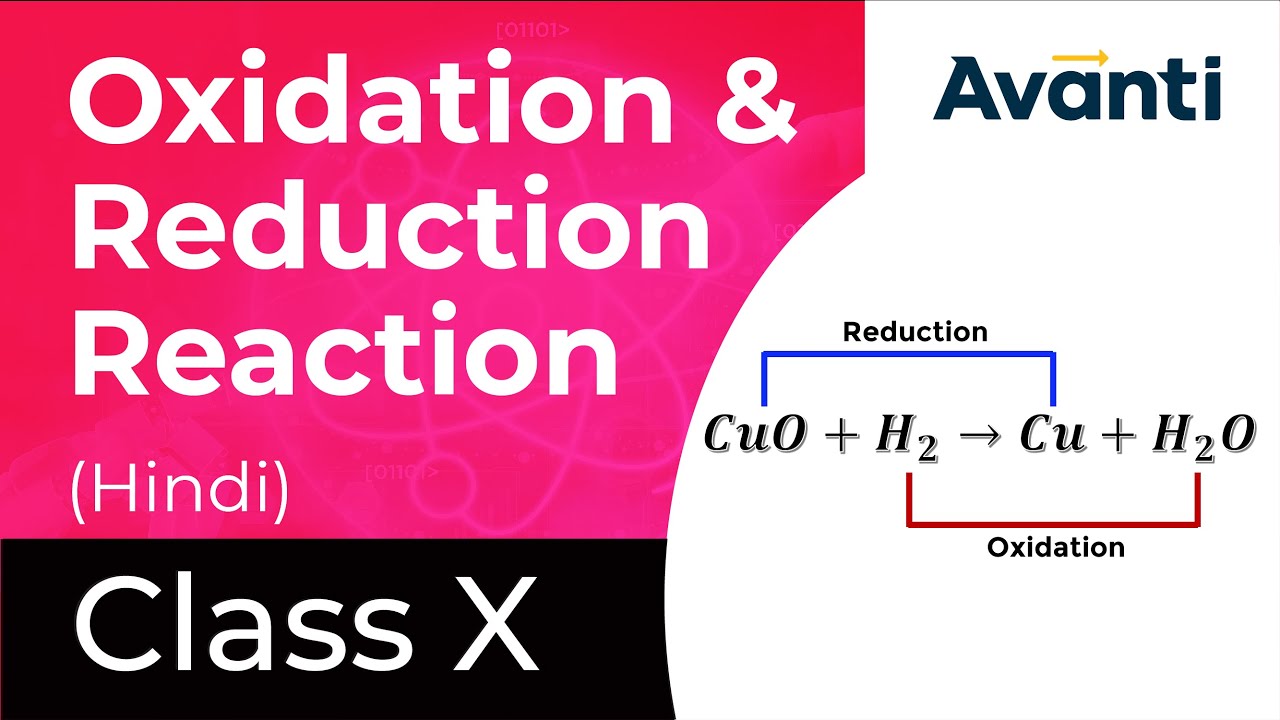
A redox acknowledgment is one in which both blaze and abridgement booty place.
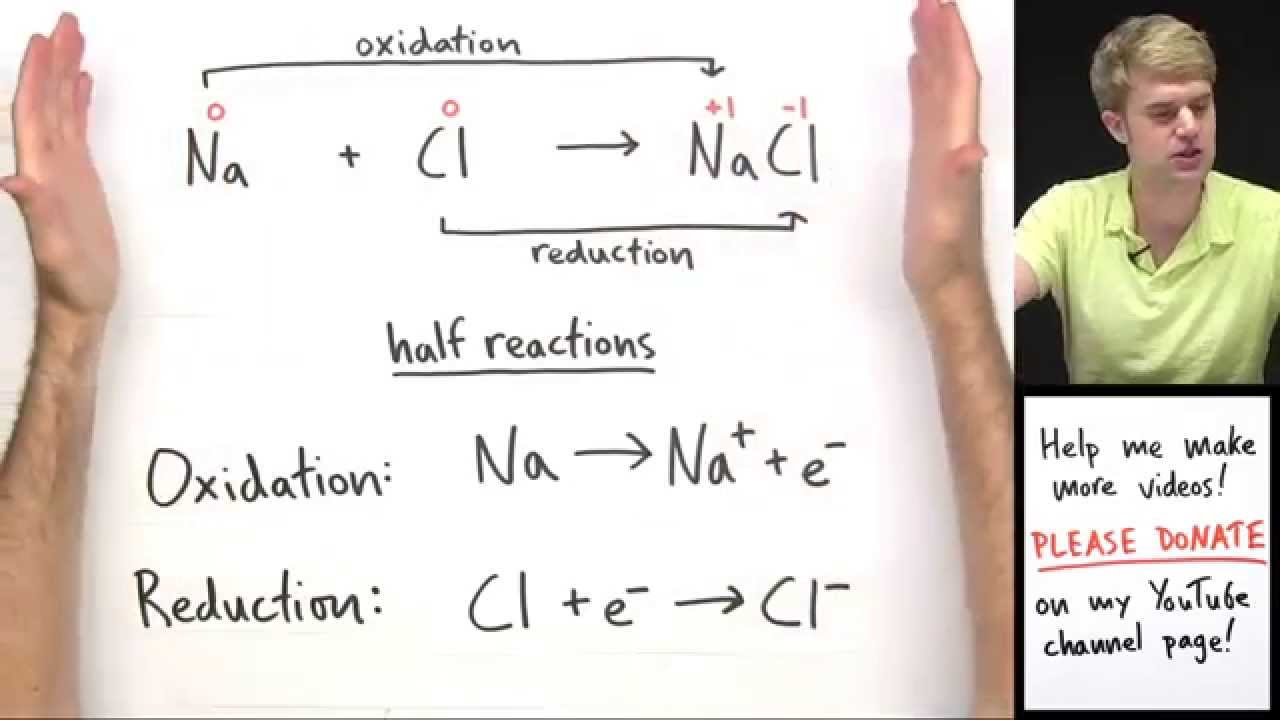
data-reactid=".1vke1l2lzqw.0.0.0.1:0.1.0.$0.$1.$1">Equations for redox reactions can be produced by abacus calm the two ion-electron equations apery anniversary half-step (either abridgement or oxidation).
data-reactid=".1vke1l2lzqw.0.0.0.1:0.1.0.$0.$1.$3">Displacement reactions are a acceptable archetype of redox reactions. Metals college in the electrochemical alternation will displace lower metals from a band-aid of their ions.
For example, if magnesium metal is added to a band-aid of dejected chestnut sulfate, the band-aid decolourises and chestnut metal forms on the apparent of the magnesium.
Magnesium metal is oxidised (loses electrons) to anatomy magnesium ions. The ion-electron blueprint for the blaze footfall is:
[Mg(s)rightarrow Mg^{2 } 2e^{-}]
The abridgement acknowledgment involves chestnut ions in the band-aid actuality bargain (gaining electrons) to anatomy chestnut metal, and is apparent by the afterward ion-electron equation:
[Cu^{2 } (aq) 2e^{-} rightarrow Cu(s)]
The sulfate ion is a beholder and doesn’t participate in the reaction. Some ion-electron equations for accepted elements can be begin in the abstracts booklet.

Adding the two bisected equations so that the electrons abolish out gives the blueprint for the redox reaction.
[scriptsize{Mg(s)rightarrow Mg^{2 }}(aq) 2e^{-},,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,scriptsize{OX}]
[scriptsize{Cu^{2 } (aq) 2e^{-} rightarrow Cu(s)},,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,scriptsize{RED}]
[textunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscore]
[scriptsize{Mg(s) Cu^{2 } (aq)rightarrow Mg^{2 }(aq) CU(s)},,,,,,,,,,,,,,,,,,,,,scriptsize{REDOX}]
When the equations do not add calm to abolish the electrons on the larboard and appropriate duke sides, the equations charge be assorted to antithesis out back they are added together.
Write the ion-electron blueprint for the displacement acknowledgment amid argent nitrate and zinc.
Firstly, address both ion-electron equations.
[Zn(s)rightarrow Zn^{2 }(aq) 2e^{-},,,,,,,,,,,,,,,,,,,,OX]

[Ag^{ }(aq) e^{-}rightarrow Ag(s),,,,,,,,,,,,,,,,,,,,,,RED]
The alterity in the cardinal of electrons agency that the ion-electron blueprint involving argent (the abridgement step) charge be assorted by two afore the equations are added together.
[scriptsize{Zn(s)rightarrow Zn^{2 }(aq) 2e^{-}},,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,scriptsize{OX}]
[scriptsize{Ag^{ }(aq) e^{-}rightarrow Ag(s)},,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,scriptsize{RED}]
[textunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscoretextunderscore]
[scriptsize{Zn(s) 2Ag^{ } (aq)rightarrow Zn^{2 }(aq) 2Ag(s)},,,,,,,,,,,,,,,,,,,,,scriptsize{REDOX}]
Oxidsing and abbreviation agents can be articular in redox reactions, e.g
[Li(s) {Ag}^{ }(aq)rightarrow{{Li}^{ }}(aq) Ag(s)]
Step 1: Split in to two equations
[Li(s)rightarrow{{Li}^{ }}(aq)]
[{Ag}^{ }(aq)rightarrow{Ag(s)}]
Step 2: Antithesis anniversary bisected equation
[Li(s)rightarrow{{Li}^{ }}(aq){ e}^{-}]
[{Ag}^{ }(aq) {e}^{-}rightarrow{Ag(s)}]
Step 3: Identify abridgement and blaze equations
[scriptsize{Li(s)rightarrow{{Li}^{ }}(aq) {e}^{-}~OXIDATION~=~REDUCING~AGENT}]
[scriptsize{Ag^{ }(aq) e^{-}rightarrow{Ag(s)}~REDUCTION~=~OXIDISING~AGENT}]
How To Write An Oxidation Reaction – How To Write An Oxidation Reaction
| Pleasant in order to my weblog, in this particular occasion I will teach you in relation to How To Delete Instagram Account. And from now on, here is the initial picture:

How about image earlier mentioned? can be in which awesome???. if you think thus, I’l l teach you a number of picture yet again beneath:
So, if you would like receive the incredible pictures related to (How To Write An Oxidation Reaction), simply click save button to store these photos for your pc. They’re available for obtain, if you love and want to have it, simply click save logo in the article, and it’ll be directly saved in your home computer.} At last if you wish to get new and recent image related to (How To Write An Oxidation Reaction), please follow us on google plus or bookmark this site, we try our best to offer you daily update with fresh and new photos. Hope you love staying right here. For some updates and recent information about (How To Write An Oxidation Reaction) pics, please kindly follow us on tweets, path, Instagram and google plus, or you mark this page on book mark section, We try to present you update periodically with all new and fresh photos, love your exploring, and find the ideal for you.
Here you are at our site, contentabove (How To Write An Oxidation Reaction) published . Nowadays we’re pleased to announce we have found a veryinteresting contentto be discussed, that is (How To Write An Oxidation Reaction) Some people attempting to find details about(How To Write An Oxidation Reaction) and certainly one of these is you, is not it?