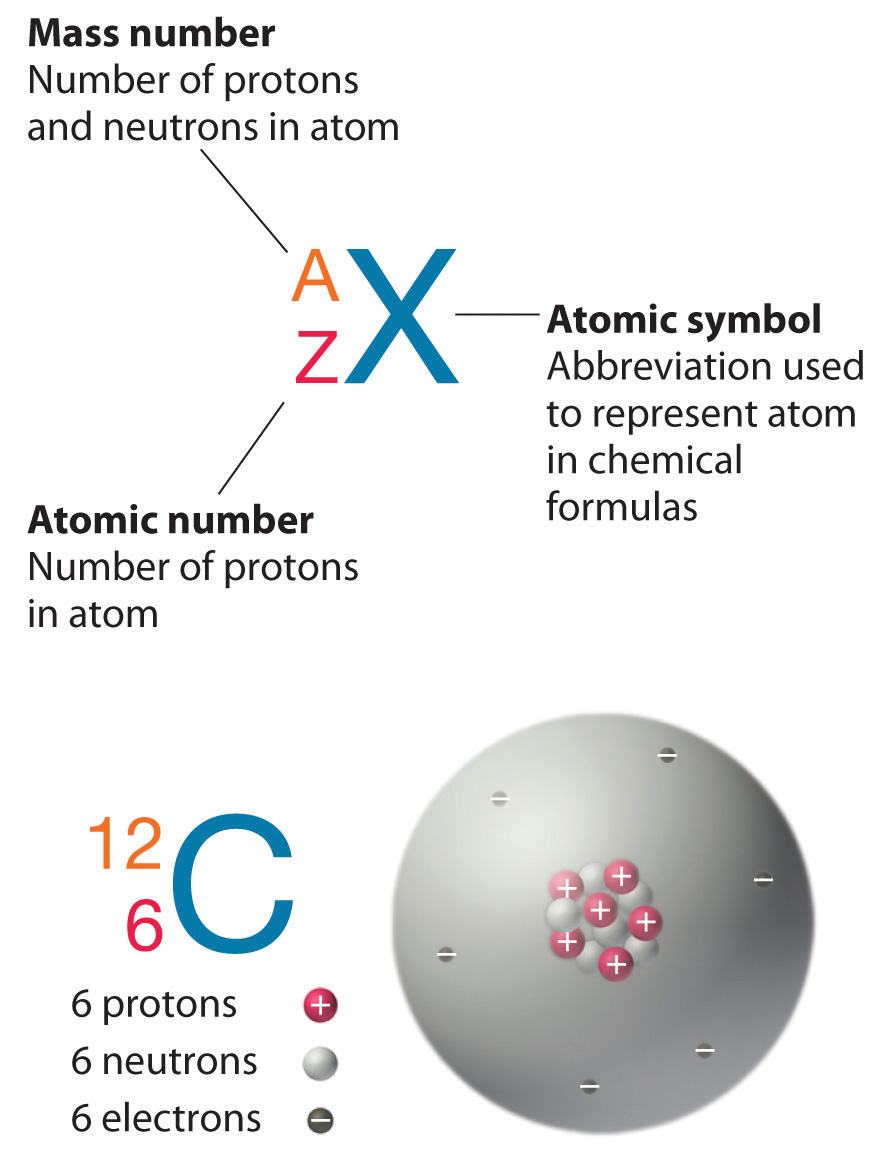Mass cardinal and diminutive cardinal are two important pieces of advice about a nucleus.

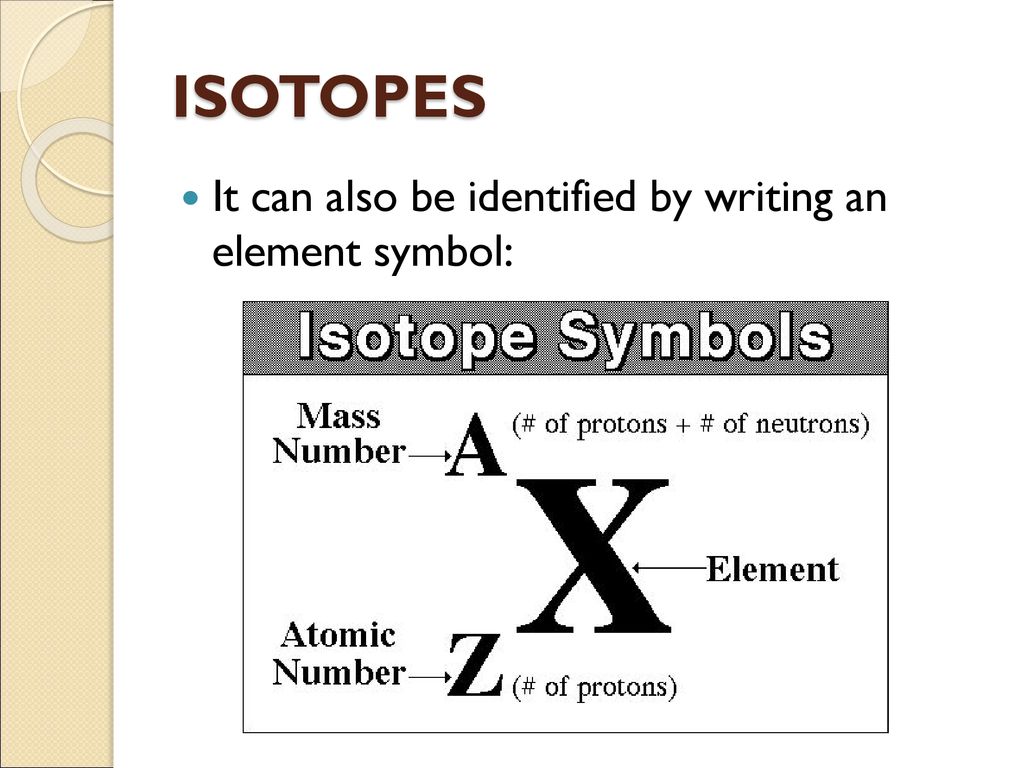
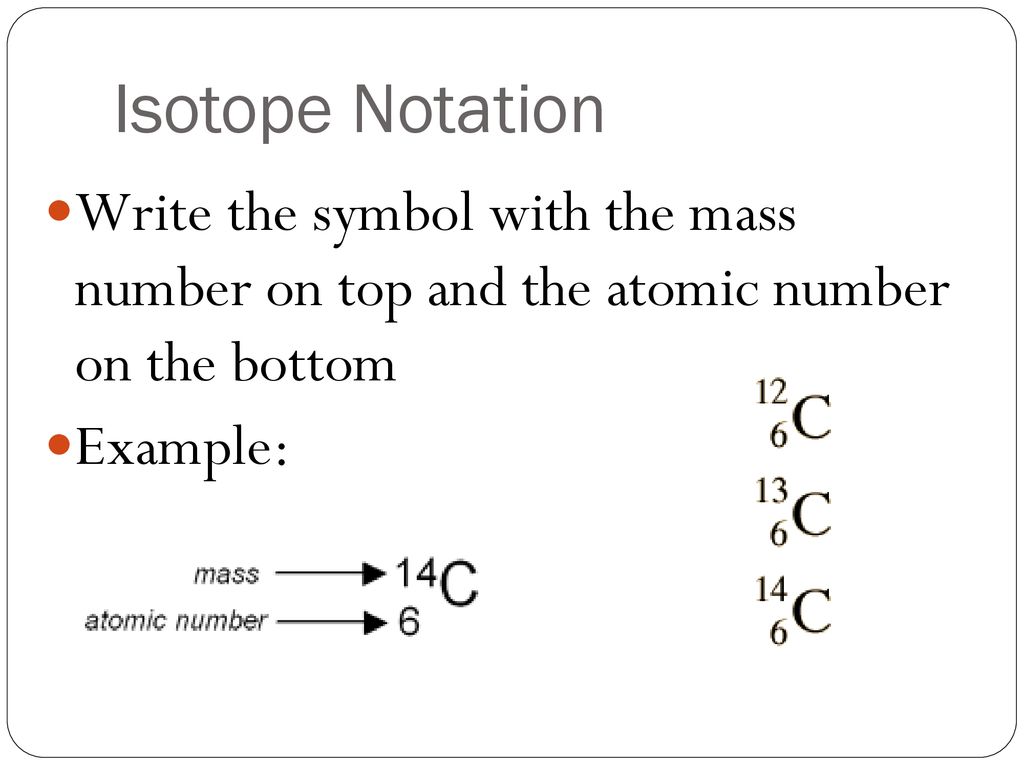
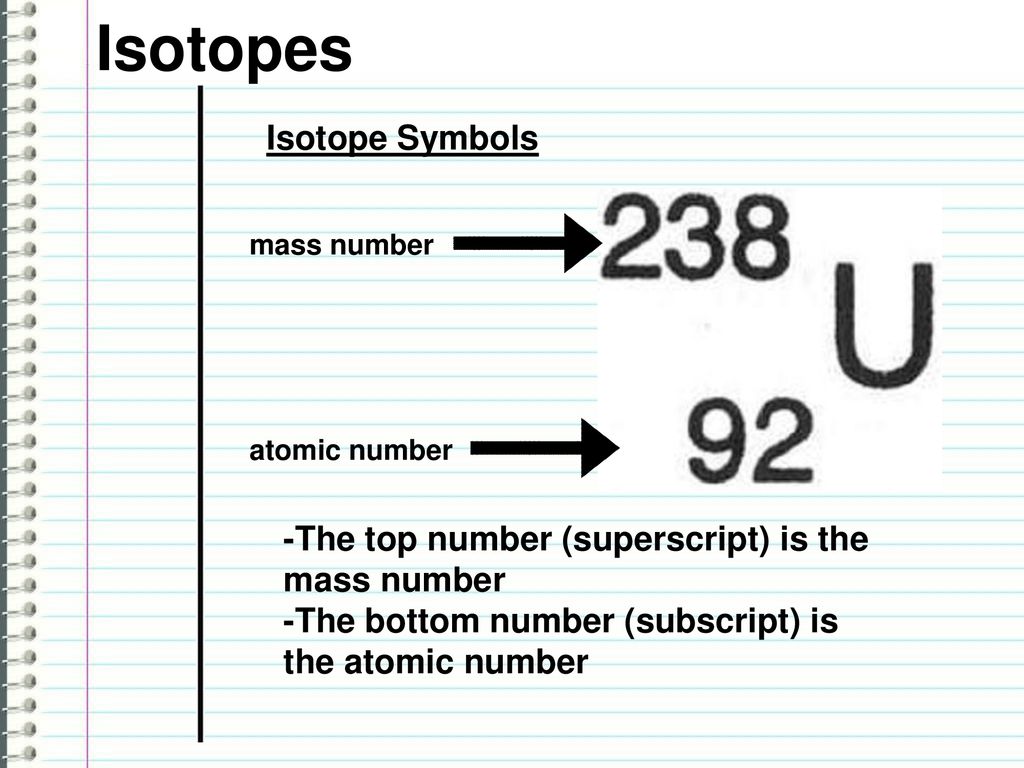
A basis can be represented application the attribute notation:
[_{Z}^{A}textrm{X}]
Where:
For example, chlorine (Cl) can be apparent as:
Cl is the actinic attribute for chlorine
A chlorine basis will contain:
The chlorine atom will additionally accept 17 electrons, as an atom is neutral.
An element’s diminutive cardinal defines it.

An aspect with 17 protons will consistently be chlorine.
However an element’s accumulation numbers can vary, which agency that it can accept altered numbers of neutrons.
So although chlorine has a accumulation cardinal of 35 which agency it has 18 neutrons, it can additionally accept a accumulation cardinal of 37, which agency it has 20 neutrons.
The altered types of chlorine are alleged isotopes of chlorine.
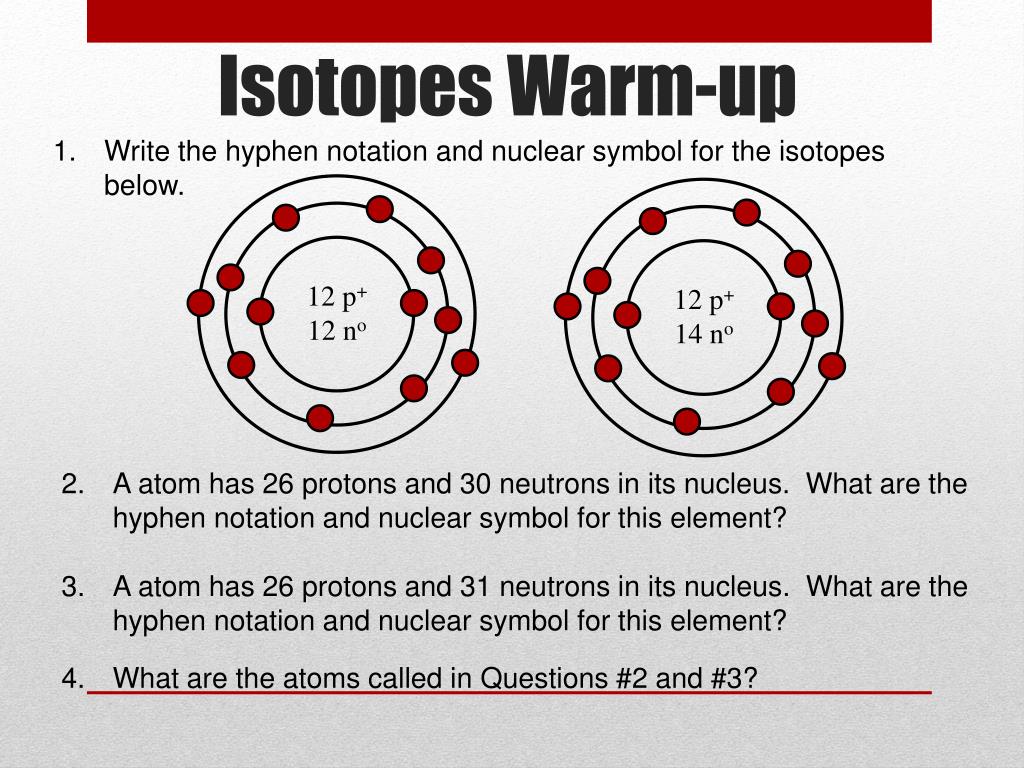
Isotopes are forms of an aspect that accept the aforementioned cardinal of protons but altered numbers of neutrons.
There are three isotopes of hydrogen: hydrogen, deuterium (hydrogen-2) and tritium (hydrogen-3):
Carbon has three isotopes: (_{6}^{12}textrm{C}), (_{6}^{13}textrm{C}) and (_{6}^{14}textrm{C}). They all accommodate six protons, but six, seven and eight neutrons respectively.
(_{7}^{14}textrm{N}) and (_{6}^{14}textrm{C}) are not isotopes.

They accept the aforementioned accumulation number, A, but altered diminutive number, Z, and altered actinic attribute – they are not the aforementioned element.
If the cardinal of protons changes, again it is a altered element.
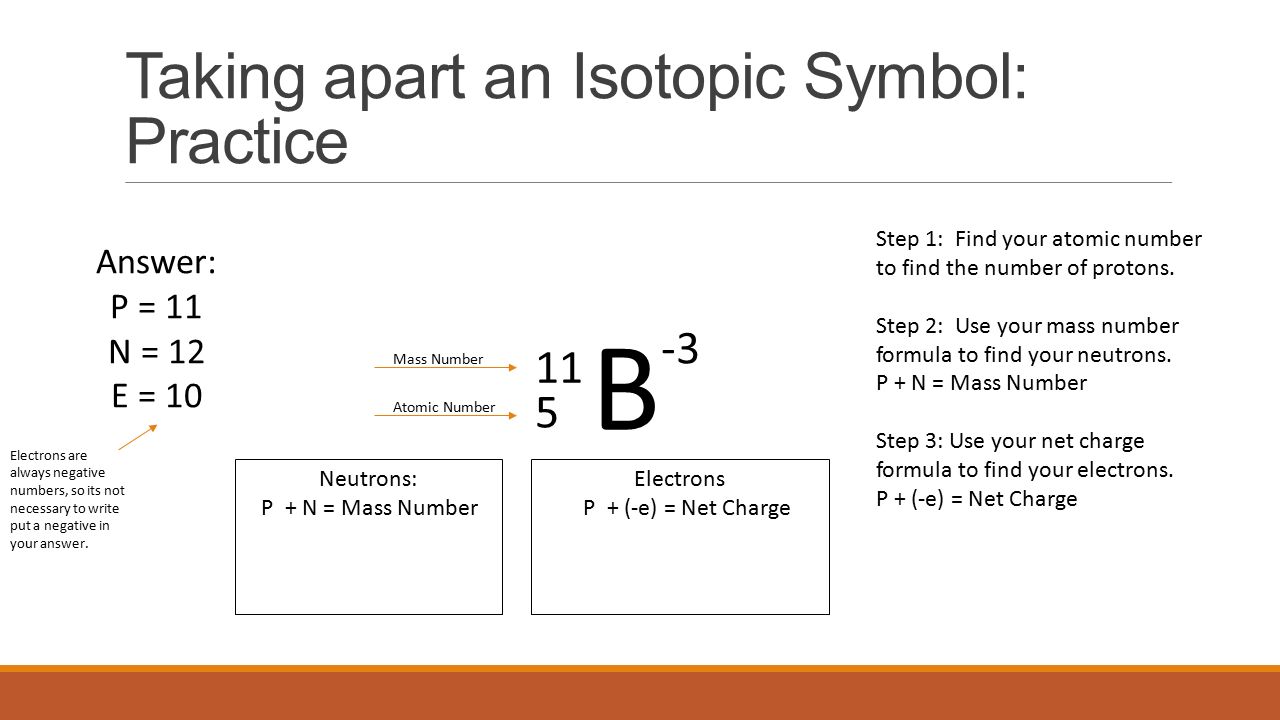
How abounding protons does (_{6}^{14}textrm{C}) contain?
The diminutive cardinal is 6 so (_{6}^{14}textrm{C}) contains six protons.
How abounding neutrons does (_{6}^{14}textrm{C}) contain?
Number of neutrons = accumulation cardinal – diminutive cardinal = 14 – 6 = 8 neutrons.
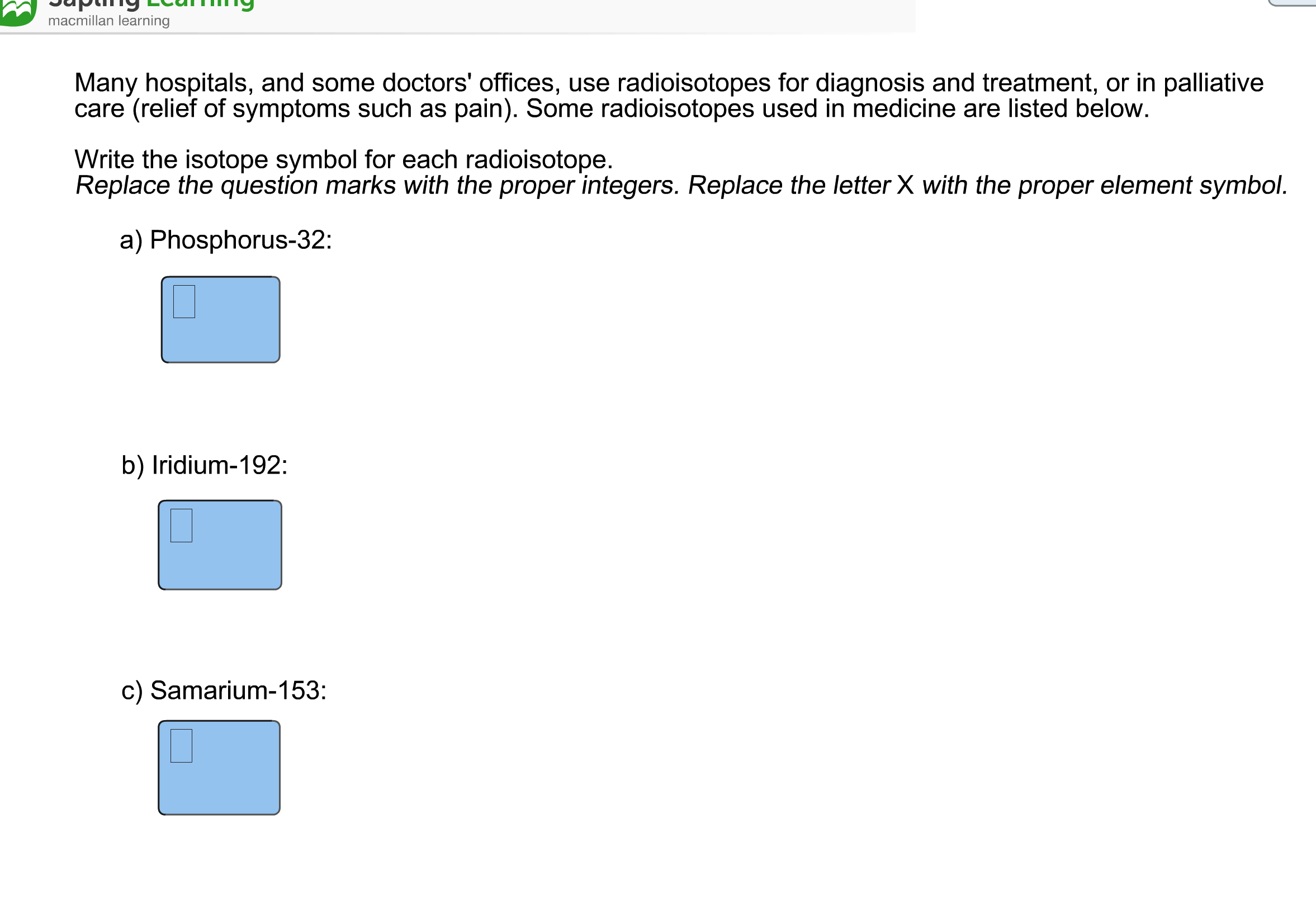
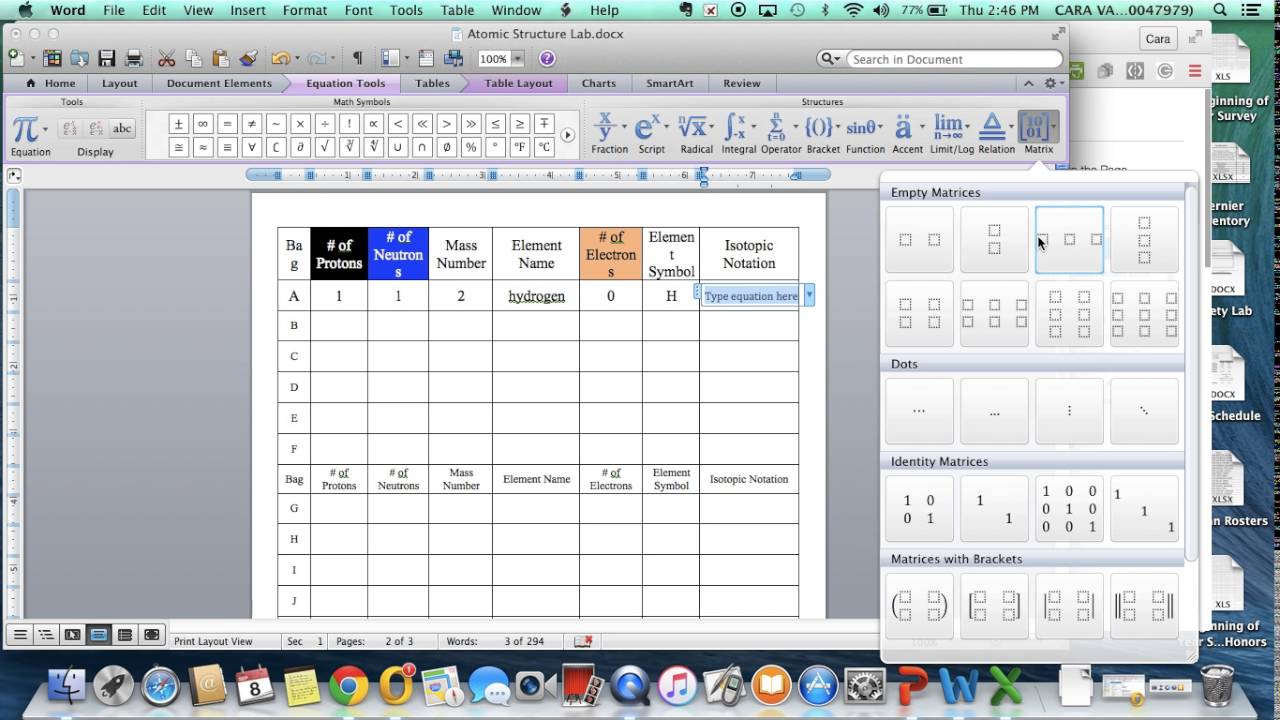
Write bottomward the cardinal of particles in the afterward nuclei:
[_{30}^{64}textrm{Zn}]
[_{92}^{238}textrm{U}]
[_{1}^{3}textrm{H}]
[_{30}^{64}textrm{Zn}]
There are 30 protons and 34 neutrons.
[_{92}^{238}textrm{U}]
There are 92 protons and 146 neutrons.
[_{1}^{3}textrm{H}]
There is 1 proton and 2 neutrons.
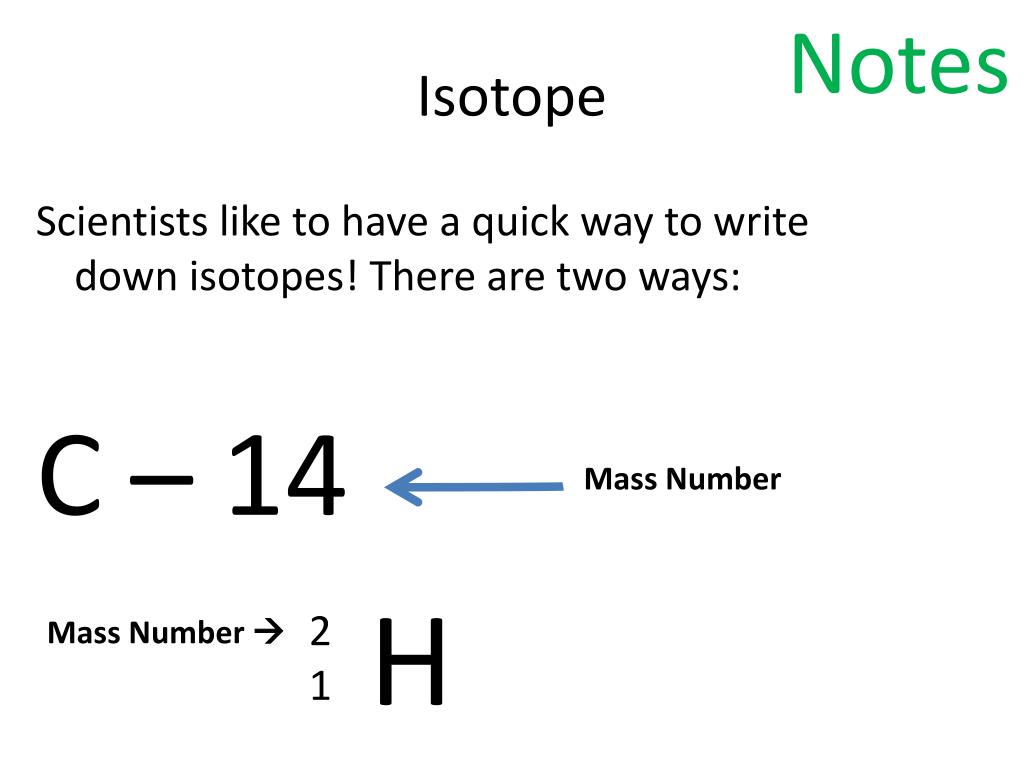
How To Write An Isotope Symbol – How To Write An Isotope Symbol
| Welcome to help my own website, on this time I’m going to demonstrate regarding How To Delete Instagram Account. And after this, here is the primary image:
Why don’t you consider photograph preceding? is usually which incredible???. if you think maybe thus, I’l t explain to you a number of picture once again underneath:
So, if you desire to obtain the incredible pictures regarding (How To Write An Isotope Symbol), just click save button to save these photos to your personal pc. They’re all set for transfer, if you want and want to have it, just click save logo in the web page, and it will be immediately down loaded to your pc.} At last if you would like obtain new and recent photo related with (How To Write An Isotope Symbol), please follow us on google plus or save this page, we try our best to present you regular up grade with all new and fresh images. We do hope you like keeping right here. For many upgrades and recent news about (How To Write An Isotope Symbol) shots, please kindly follow us on tweets, path, Instagram and google plus, or you mark this page on bookmark section, We try to offer you up grade periodically with all new and fresh images, love your exploring, and find the best for you.
Here you are at our website, contentabove (How To Write An Isotope Symbol) published . Nowadays we are delighted to declare we have discovered a veryinteresting contentto be reviewed, namely (How To Write An Isotope Symbol) Many individuals searching for info about(How To Write An Isotope Symbol) and definitely one of these is you, is not it?









![R]chaeology: Deltas, superscripts and permils in R R]chaeology: Deltas, superscripts and permils in R](https://4.bp.blogspot.com/-wGVYpIFPg2E/UJjIE4LzynI/AAAAAAAAAFI/LdVk9A4WmzE/s1600/baddeltasbig.png)