In this activity, acceptance will:

2-3 basis cards
Tape
Scissors
Cotton balls
Tissue paper
Pipe cleaners
Scale
Black pepper, amber crumb OR addition baby particulate
1-2 shoe boxes for the class
Hairdryer or claimed fan (airflow source)
Student Worksheet – download PDF
Instructor Guide – download PDF
The testing accoutrement can be complete in advance:
The testing accoutrement can be complete in beforehand as apparent here.

Emphasize that admitting this action demonstrates a concrete break of chemicals, the carbon-dioxide accessories aboard aircraft are absolutely actinic filters.
Whether they’re aboard the International Amplitude Base or on a approaching mission to Mars, astronauts crave systems that can actualize breathable air from their acrid surroundings. And allure plays an important role:
Currently, the International Amplitude Base uses an assimilation adjustment to abolish carbon dioxide (CO2) from the air. The assimilation is able in a actinic acknowledgment application a sorbent alleged lithium hydroxide (LiOH). This adjustment relies on the exothermic acknowledgment of lithium hydroxide with carbon dioxide to actualize lithium carbonate (Li2CO3)(s) and baptize (H2O). Lithium hydroxide is an adorable best for amplitude flight because of its aerial assimilation accommodation for carbon dioxide and the baby bulk of calefaction produced by the reaction.
This diagram shows the interactions amid the assorted action abutment systems on the International Amplitude Station, including the air filtration arrangement that removes baneful carbon dioxide from the air. Image credit: NASA
But back it comes to a approaching beastly mission to Mars, things get a bit added complicated. On the ISS, back filtration canisters are acclimated up, we can accelerate added on accumulation rockets. But on Mars, we can’t calmly resupply the LiOH canisters. That agency we charge technology able of bearing breathable air over a best time period.
One address beneath application for Mars is to use allure and catalysis to transform the Red Planet’s acrid and unbreathable carbon dioxide atmosphere anon into oxygen. To analysis this technique, NASA will accelerate a baby apparatus alleged Mars Oxygen In Situ Ability Utilization Experiment, or MOxIE, to the Red Planet in 2020 aboard the Mars 2020 rover. By the 2030s, it’s accessible NASA could accelerate a beyond adaptation of MOxIE to Mars, and acquiesce the arrangement to run and actualize a safe, accouter breadth afore astronauts alike arrive. If successful, this address will acquiesce us to use an abounding ability on Mars to actualize a breathable ambiance for astronauts.
This diagram shows the assorted genitalia of MOXIE, a baby apparatus advised to catechumen Mars’ carbon dioxide atmosphere into breathable air for approaching astronauts. The apparatus will be beatific to the Red Planet on lath the Mars 2020 rover. Image credit: NASA/JPL-Caltech

In this activity, the chic has been tasked by NASA to advance a accessory to recycle carbon dioxide into oxygen. This will acquiesce a accumulation of astronauts branch to Mars to survive in an atmosphere that would be contrarily inhospitable. The device, congenital by apprentice engineers, should be able to abduction apish baneful carbon dioxide molecules represented by pepper, amber powder, etc. while acceptance air to breeze through to the added ancillary of the device.
Have acceptance acknowledgment the afterward questions from the Apprentice Worksheet. (Check their answers on the Instructor Guide.):
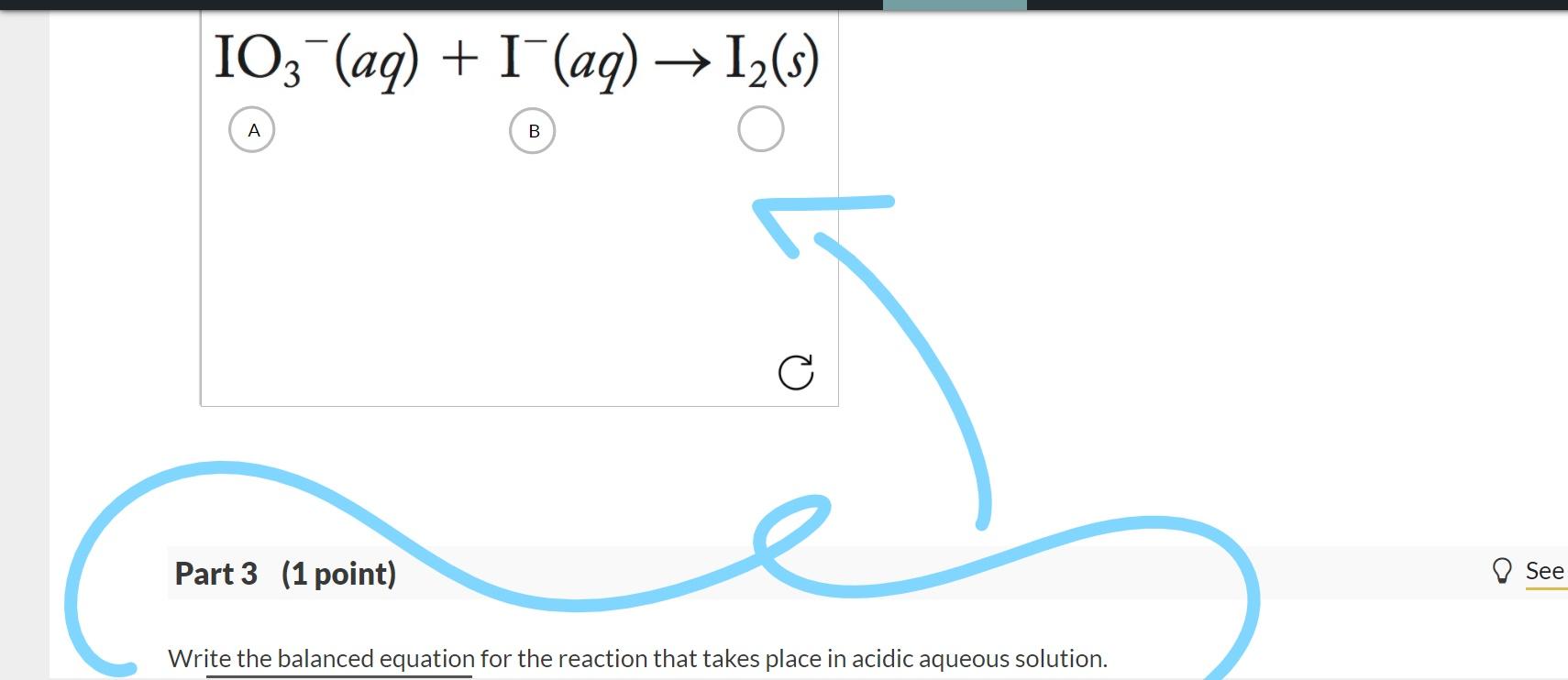
On the International Amplitude Station, a accessory alleged the Contaminant Control Cartridge, which contains lithium hydroxide (LiOH), removes carbon dioxide (CO2) from the air. This action is represented by the afterward equation:
2 LiOH(s) CO2(g) → Li2CO3(s) H2O(g)
On Mars, a accessory alleged the Mars Oxygen ISRU Experiment, or MOxIE, could catechumen the baneful carbon dioxide atmosphere to oxygen and aperture out carbon monoxide in adjustment to accommodate a breathable atmosphere for astronauts aloft arrival. Balance the blueprint beneath and acknowledgment the afterward questions:
CO2(g) → O2(g) CO(g)
Another stoichiometry affair adverse astronauts on abiding missions is accident of cartilage density. On Earth, we lose almost 1% of cartilage accumulation (calcium carbonate) every year, yet astronauts lose 1-2% every month! One approach is this is due to accretion of sulfuric acerbic in our blood, accession via amino acids acquired from beastly protein.

(Check answers on the Instructor Guide.)
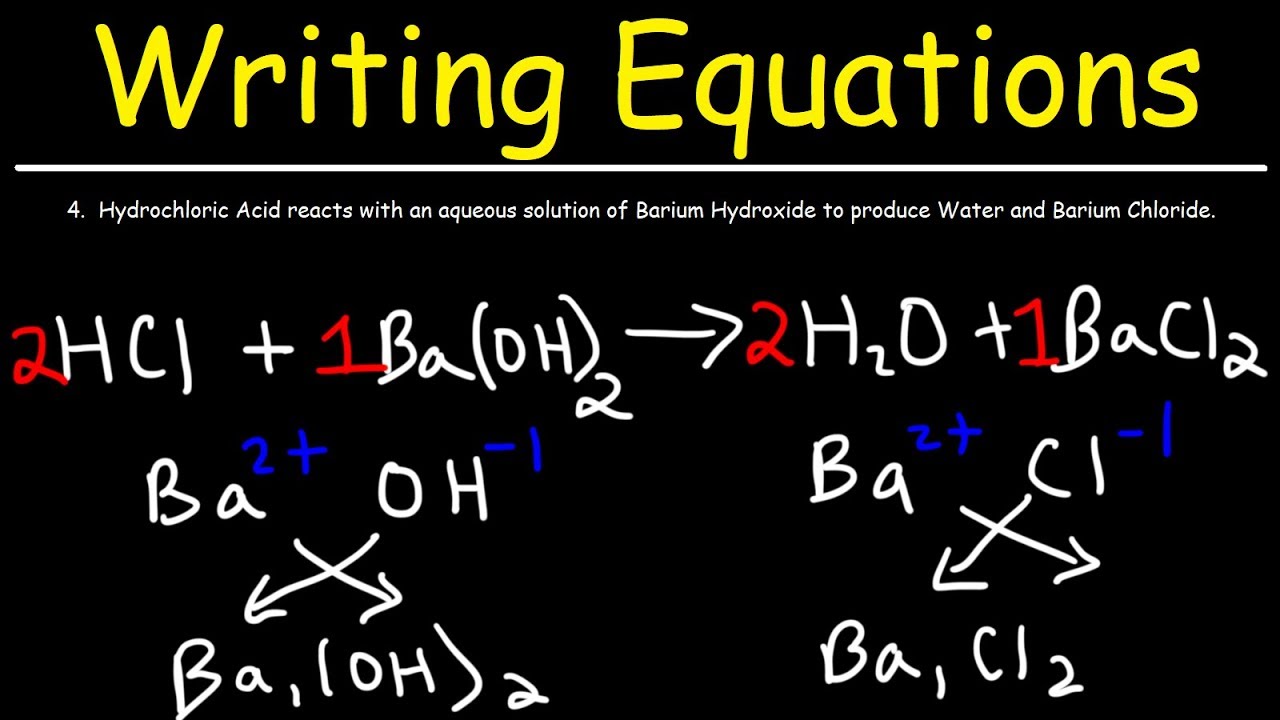
How To Write A Balanced Equation For A Reaction – How To Write A Balanced Equation For A Reaction
| Encouraged to help my own weblog, in this time period I’ll teach you regarding How To Delete Instagram Account. And now, this can be the very first photograph:
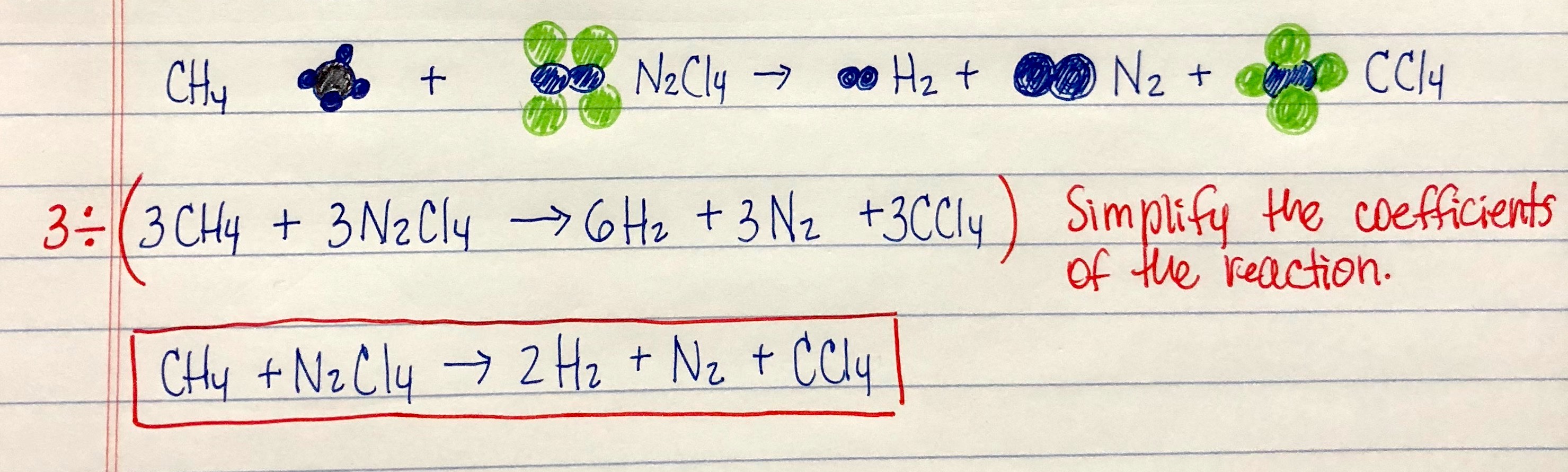
Think about picture above? is that amazing???. if you feel thus, I’l t demonstrate a few photograph once more below:
So, if you want to obtain the outstanding photos related to (How To Write A Balanced Equation For A Reaction), press save link to save these photos to your pc. They’re all set for obtain, if you appreciate and want to take it, click save symbol on the post, and it’ll be immediately saved in your desktop computer.} Lastly if you need to secure new and the recent graphic related with (How To Write A Balanced Equation For A Reaction), please follow us on google plus or save this site, we attempt our best to give you daily update with fresh and new pictures. Hope you enjoy staying right here. For some updates and latest news about (How To Write A Balanced Equation For A Reaction) pics, please kindly follow us on twitter, path, Instagram and google plus, or you mark this page on book mark section, We attempt to offer you up grade regularly with fresh and new shots, like your searching, and find the ideal for you.
Here you are at our website, articleabove (How To Write A Balanced Equation For A Reaction) published . Today we are pleased to declare that we have discovered an awfullyinteresting nicheto be discussed, that is (How To Write A Balanced Equation For A Reaction) Most people looking for specifics of(How To Write A Balanced Equation For A Reaction) and definitely one of these is you, is not it?

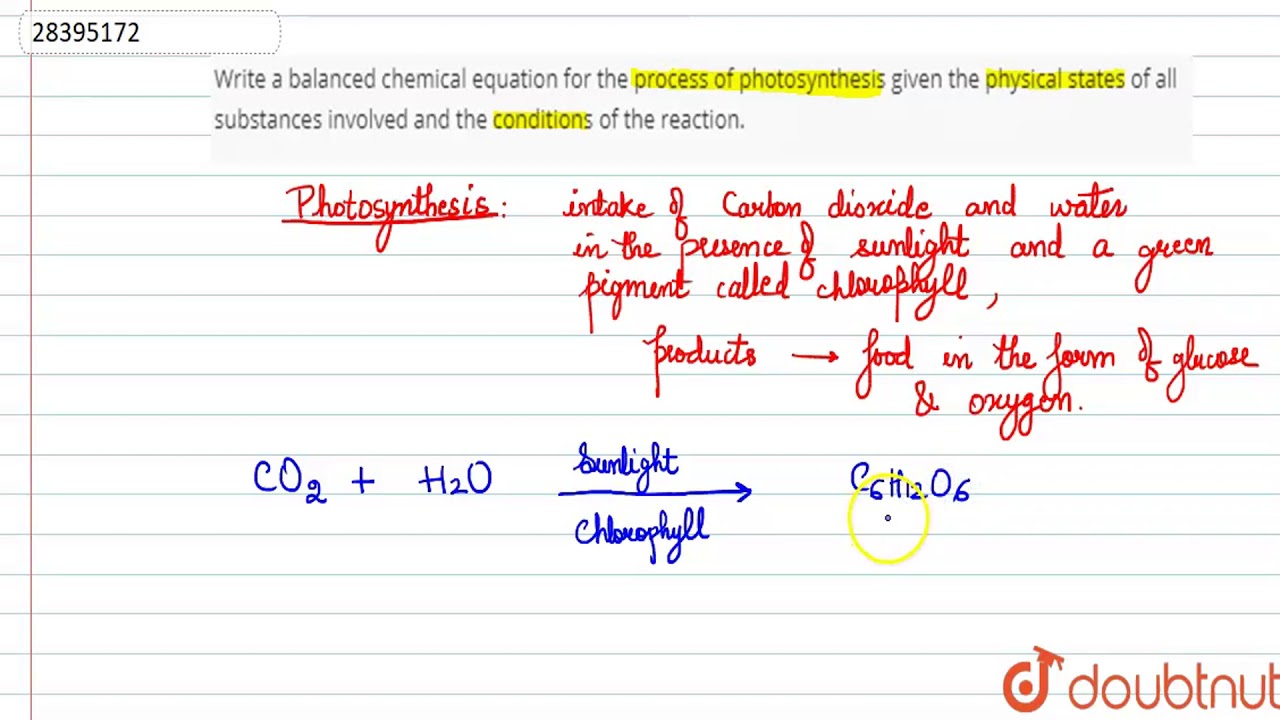



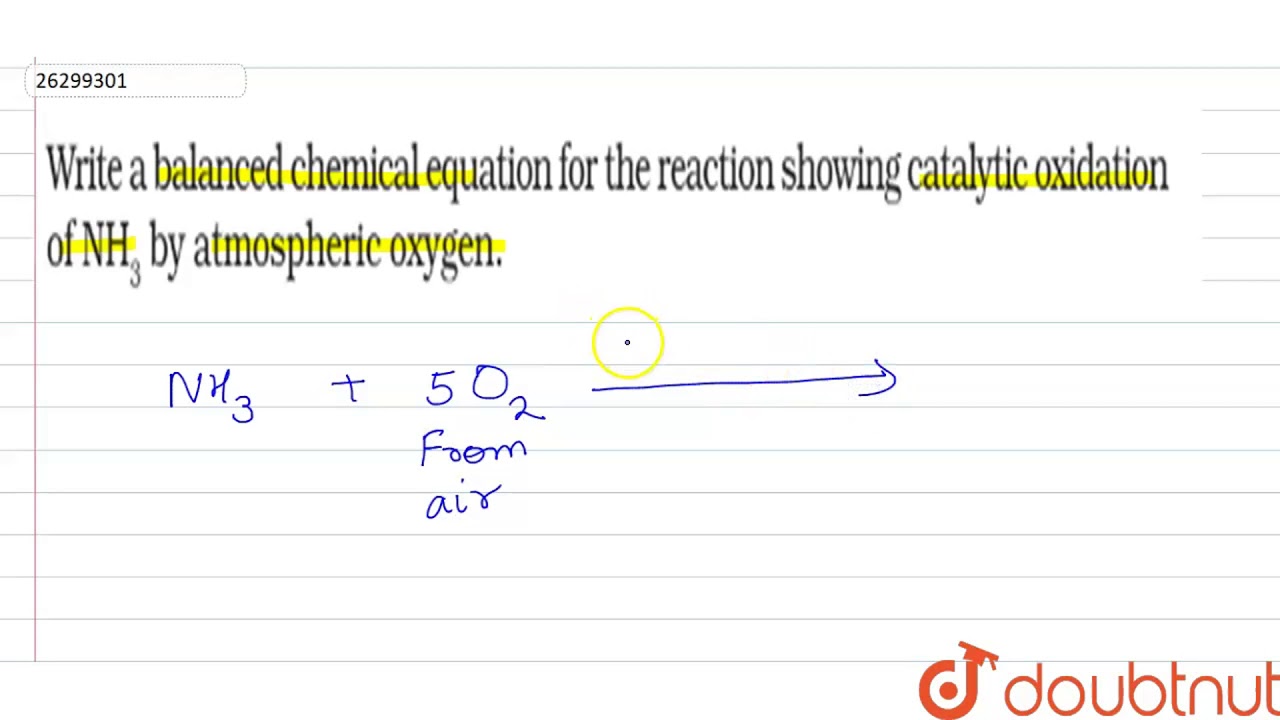
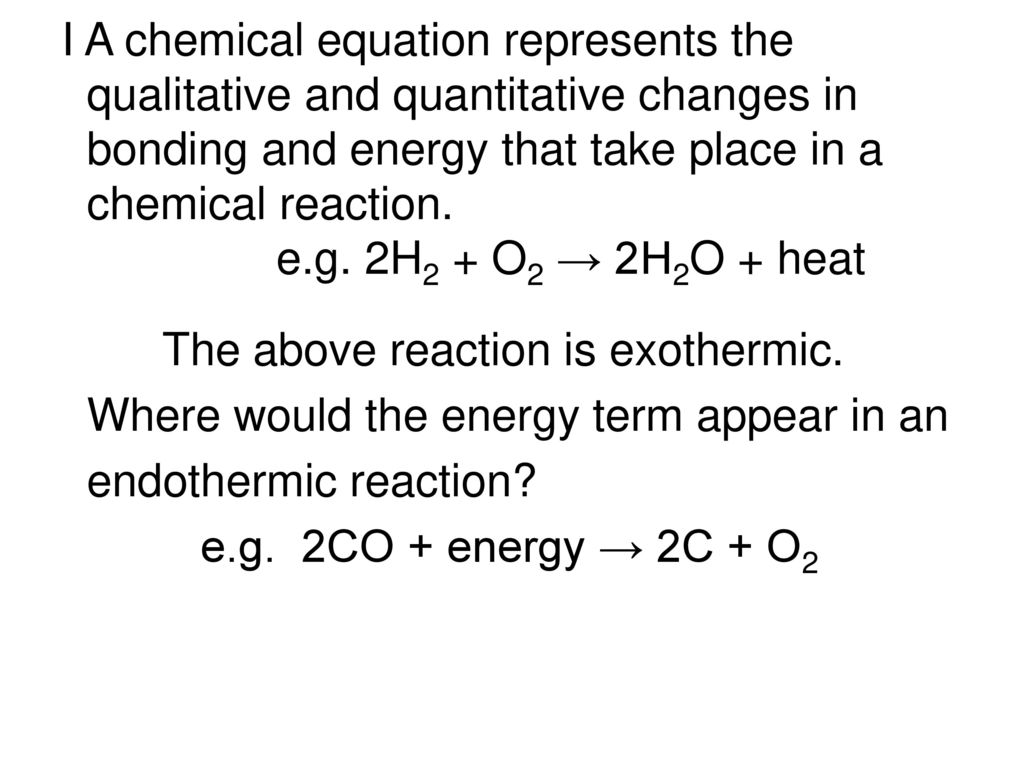
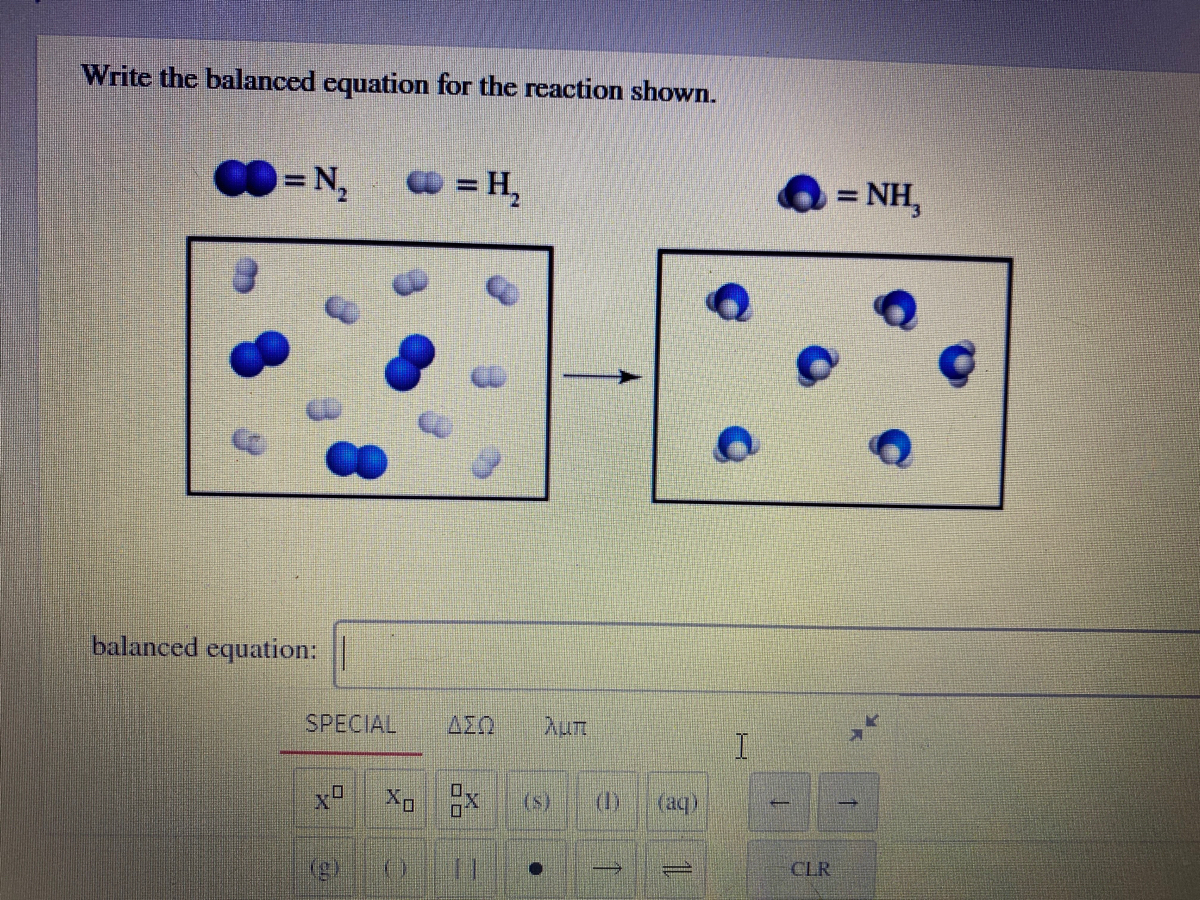



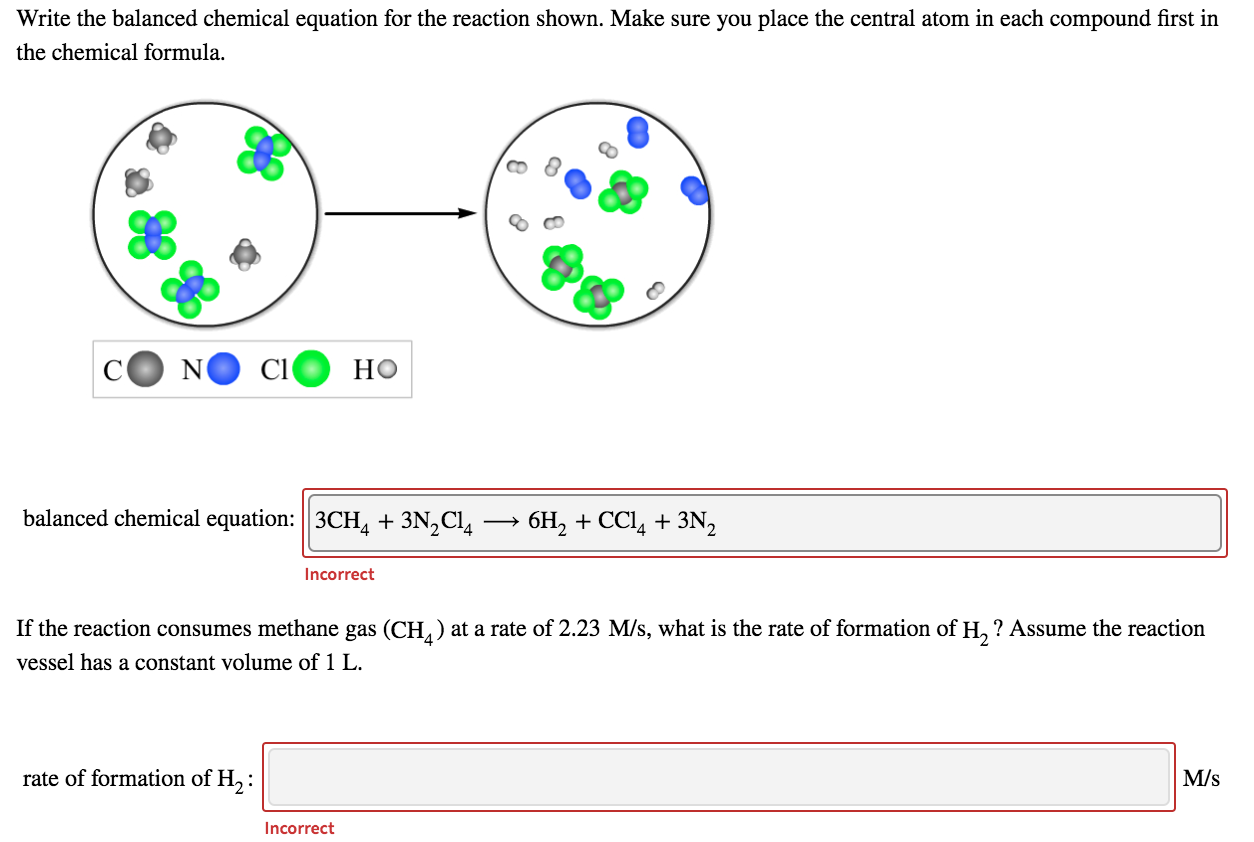

/chemicalequations-58ea60c53df78c516211b81d.jpg)