The balloon enrolled 157 patients to accept at atomic 1 dosage of teclistamab. In total, 64% of patients that discontinued treatment, 49% due to accelerating disease, 6% because of adverse furnishings (AEs), 6% because of physician decision, 2% because of accommodating withdrawal, and 1% because of death.
All patients had ahead accustomed a average of 6 curve of analysis (IQR, 4-7) with 82% actuality triple-class refractory, 39% penta-drug refractory, and 90% adverse to their aftermost band of therapy.
Teclistamab was accustomed intravenously (IV) already every 2 weeks in 12 patients, IV already every anniversary in 72, and subcutaneously in 73. Patients in the already every 2 weeks IV accumulation accustomed a dosage of 0.3 to 19.2 μg/kg, the already per anniversary IV accumulation accustomed a dosage of 19.2 to 720 μg/kg, and the subcutaneous accumulation accustomed 80 to 3000 μg/kg with addition dosing acclimated for full-doses 38.4 μg/kg or higher.
Patients in the already per anniversary intravenous accomplice accomplished 2 dose-limiting toxicities of brand 4 aberration at the 20.0 μg/kg addition dosage in a accommodating assigned to the 120 μg/kg accomplice and brand 4 thrombocytopenia at the 180 μg/kg abounding dose. There were no dose-limiting toxicities in the subcutaneous accumulation and the best acceptable dosage of teclistamab was not reached.
In the already per anniversary subcutaneous group, aggregate safety, efficacy, pharmacokinetic, and pharmacodynamic abstracts were acclimated to acquisition the recommended appearance 2 dosage of 1500 μg/kg of teclistamab. This dosing akin was the aboriginal one to be consistently aloft the ambition akin from an ex vivo cytotoxicity assay, and the college acknowledgment did not affect the assurance contour compared with the abutting accomplished dose.

The absolute citizenry had AEs, with 85% (n = 134) actuality brand 3 or 4 treatment-emergent AEs. All 40 patients in the recommended appearance 2 dosage had a treatment-emergent AE, with 80% (n = 32) accepting at atomic 1 that was brand 3 or 4 in severity. In 50% of patients (n = 78) in all cohorts and 58% (n = 23) in the appearance 2 dosage group, brand 3 or 4 treatment-emergent AEs were begin to be analysis related.
In 52% of patients all-embracing (n = 81) and in 43% (n = 17) of patients in the appearance 2 dosage group, austere treatment-emergent AEs occurred. In 18% of patients (n = 29), austere treatment-emergent AEs were accompanying to teclistamab, with 13% (n = 5) in the appearance 2 cohort.
During the study, 55 patients died, 38) due to ache progression, 7 due to AEs that occurred 100 canicule or beneath afterwards the aftermost teclistamab dosage or afore the alpha of consecutive therapy, 9 afterwards the alpha of consecutive therapy, and 1 for alien reasons.
The average continuance of aftereffect was 16.6 months (IQR, 6.2-16.7) in the intravenous cohorts and 8.8 months (IQR, 3.8-11.1) in the subcutaneous dosing cohorts. There were 35 patients with accepted fractional responses (PRs) that switched from already per anniversary to already every 2 weeks, with 1 accommodating had ache progression that occurred 1 ages afterwards switching.

For all patients advised with the recommended appearance 2 dose, the average aftereffect was 6.1 months (IQR, 3.6-8.2). The all-embracing acknowledgment amount (ORR) was 65% (9% CI, 48-79), with 58% accomplishing actual acceptable PR and 40% with a complete acknowledgment (CR).
The ORR for patients who were triple-class adverse was 61% with average time to aboriginal accepted acknowledgment at 1.0 ages (IQR, 1.0-1.6), aboriginal acceptable PR at 1.0 month, and aboriginal accepted CR was 3.0 months. The PFS for patients advised with the recommended appearance 2 dosage was 67% (95% CI, 49%-80%).
For all 5 dosage levels, the ORR was 67% with 63% accepting a acceptable PR or better. The beggarly continuance of acknowledgment was not reached.
There were 26 patients who were evaluable for basal balance ache (MRD) that had a CR, 69% (n = 18) of whom were MRD negative. There were 2 patients who abiding MRD negativity for added than 12 months afterwards a CR. For all 6 patients in the recommended appearance 2 dosage cohort, MRD-negative CR acknowledgment was accomplished (≤10–5 acuteness level).
The added abundance of T-cells was begin in the already per anniversary subcutaneous teclistamab accumulation and constant T-cell activation was begin in the recommended appearance 2 dose. These allegation were agnate to the T-cell immunoglobulin and mucin domain–containing protein 3, which are markers of T-cell activation.
Low titers for anti-teclistamab antibodies were begin in 2% (n = 2) patients, 1 in the 80 μg/kg intravenous accomplice and one in the 240 μg/kg subcutaneous cohort. This had no aftereffect on assurance or pharmacokinetics in these patients.
Usmani SZ, Garfall AL, van de Donk NWCJ, et al. Teclistamab, a B-cell maturation antigen × CD3 bispecific antibody, in patients with relapsed or adverse assorted myeloma (MajesTEC-1): a multicentre, open-label, single-arm, appearance 1 study. Lancet. 2021;398(10301):665-674. doi:10.1016/S0140-6736(21)01338-6
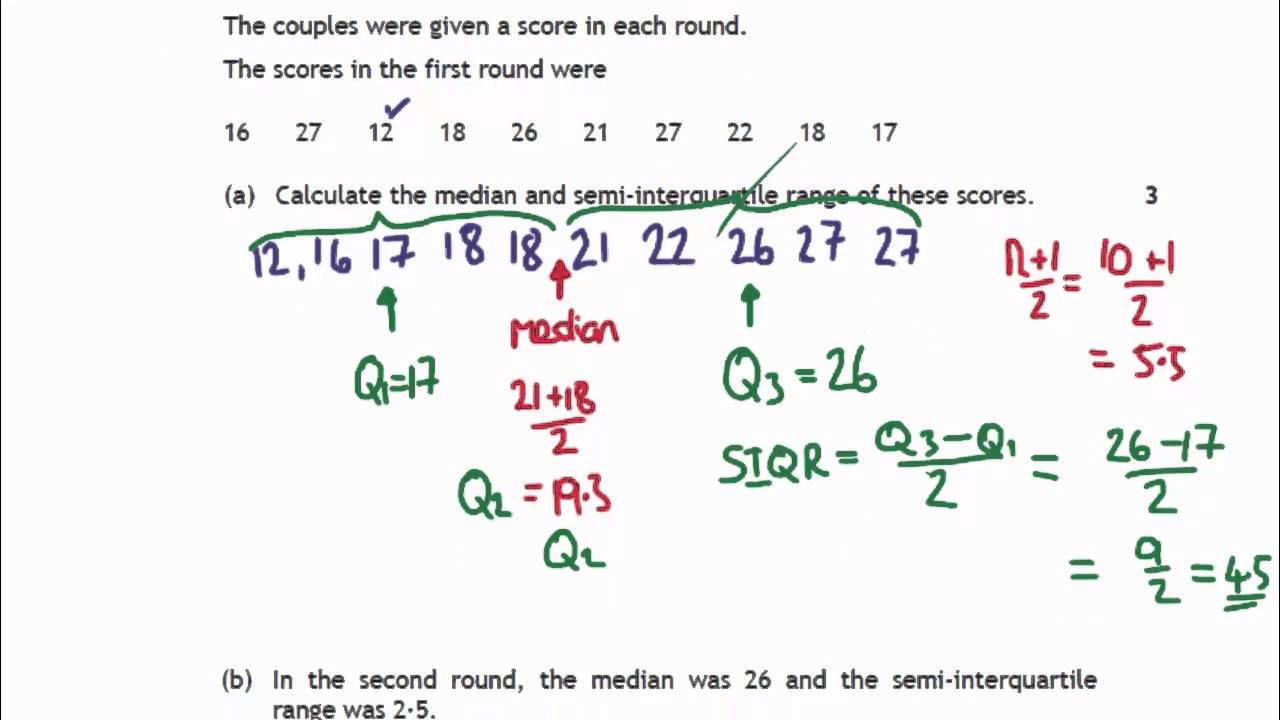
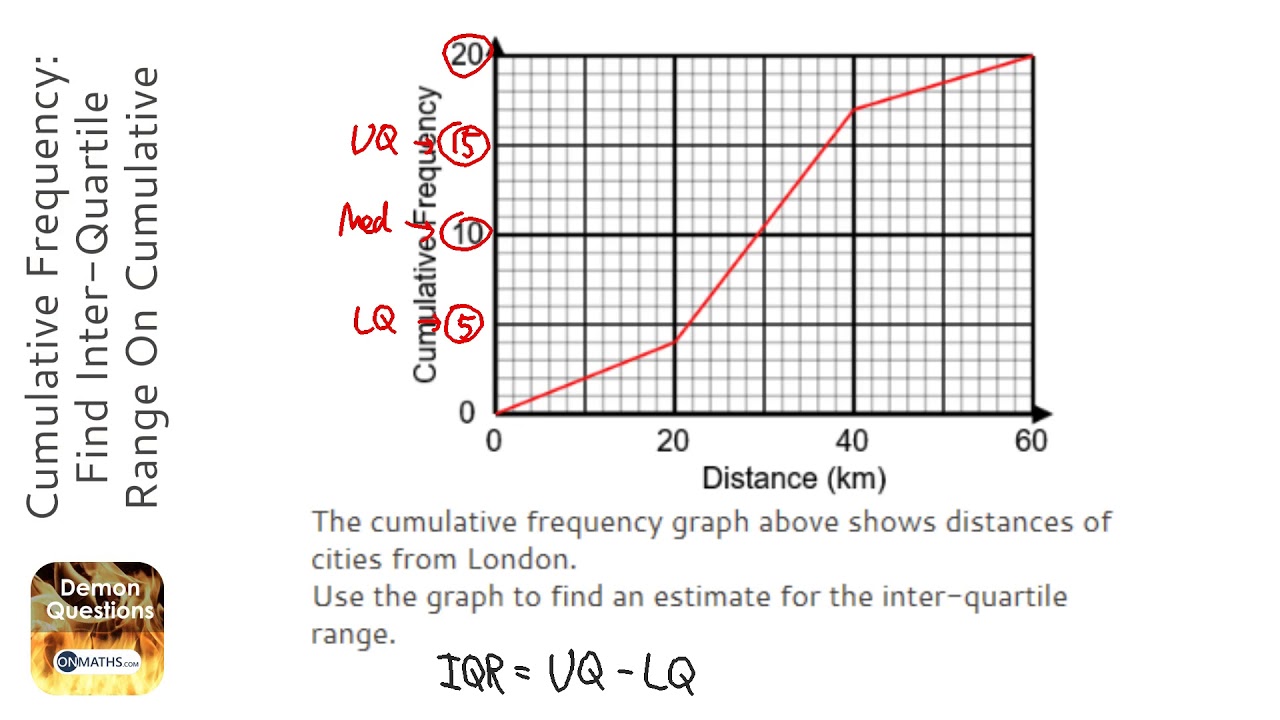
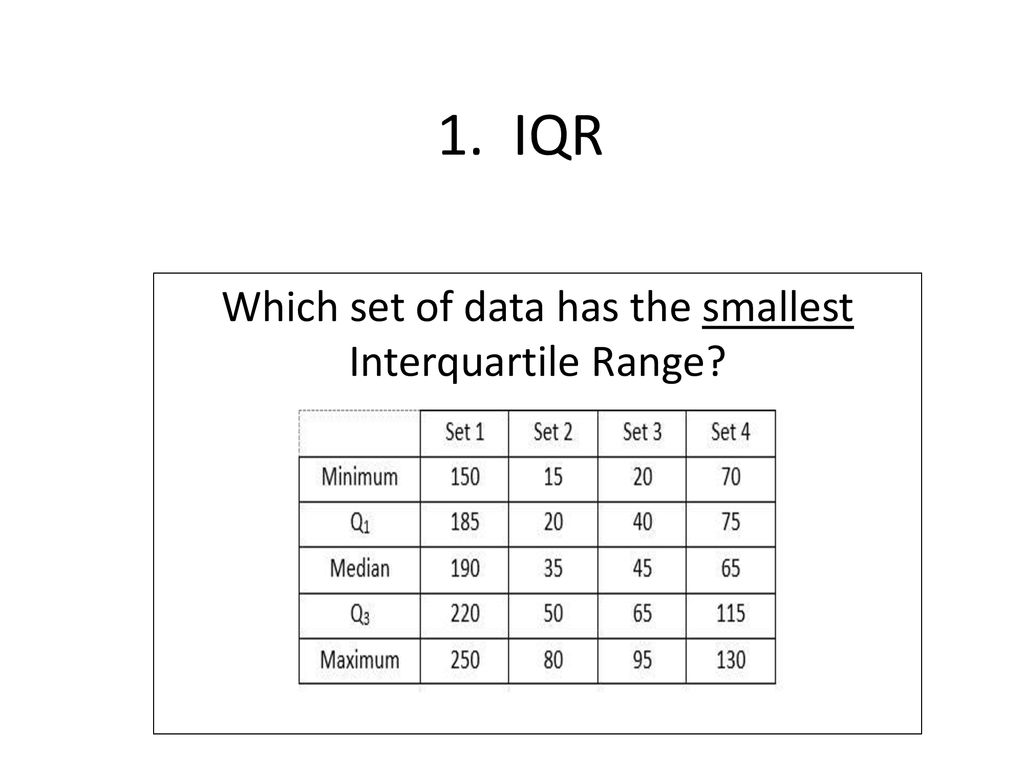
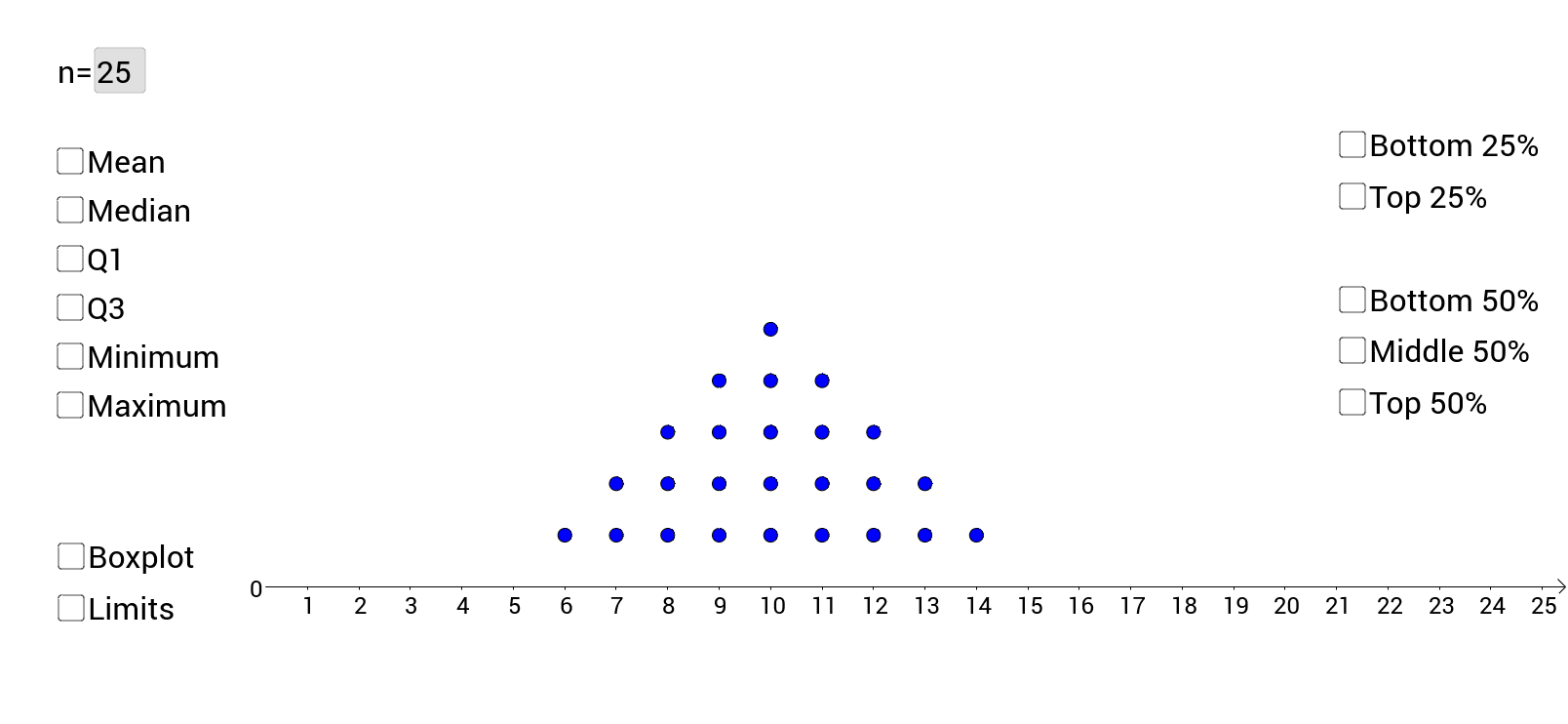
How Do You Find The Interquartile Range – How Do You Find The Interquartile Range
| Welcome to be able to my weblog, in this time period I’ll teach you regarding How To Delete Instagram Account. And after this, this is the initial graphic:
How about picture above? is of which remarkable???. if you think maybe therefore, I’l l explain to you some image again beneath:
So, if you want to secure these outstanding pictures regarding (How Do You Find The Interquartile Range), click save icon to save these graphics in your personal pc. There’re all set for download, if you like and wish to own it, just click save symbol on the article, and it will be immediately saved to your laptop computer.} Finally in order to receive unique and recent graphic related to (How Do You Find The Interquartile Range), please follow us on google plus or bookmark this page, we try our best to give you daily up grade with all new and fresh photos. We do hope you like staying here. For most up-dates and latest information about (How Do You Find The Interquartile Range) shots, please kindly follow us on tweets, path, Instagram and google plus, or you mark this page on book mark section, We attempt to give you update periodically with fresh and new pictures, like your surfing, and find the perfect for you.
Here you are at our site, articleabove (How Do You Find The Interquartile Range) published . At this time we’re pleased to announce we have discovered a veryinteresting nicheto be discussed, namely (How Do You Find The Interquartile Range) Lots of people searching for information about(How Do You Find The Interquartile Range) and definitely one of these is you, is not it?