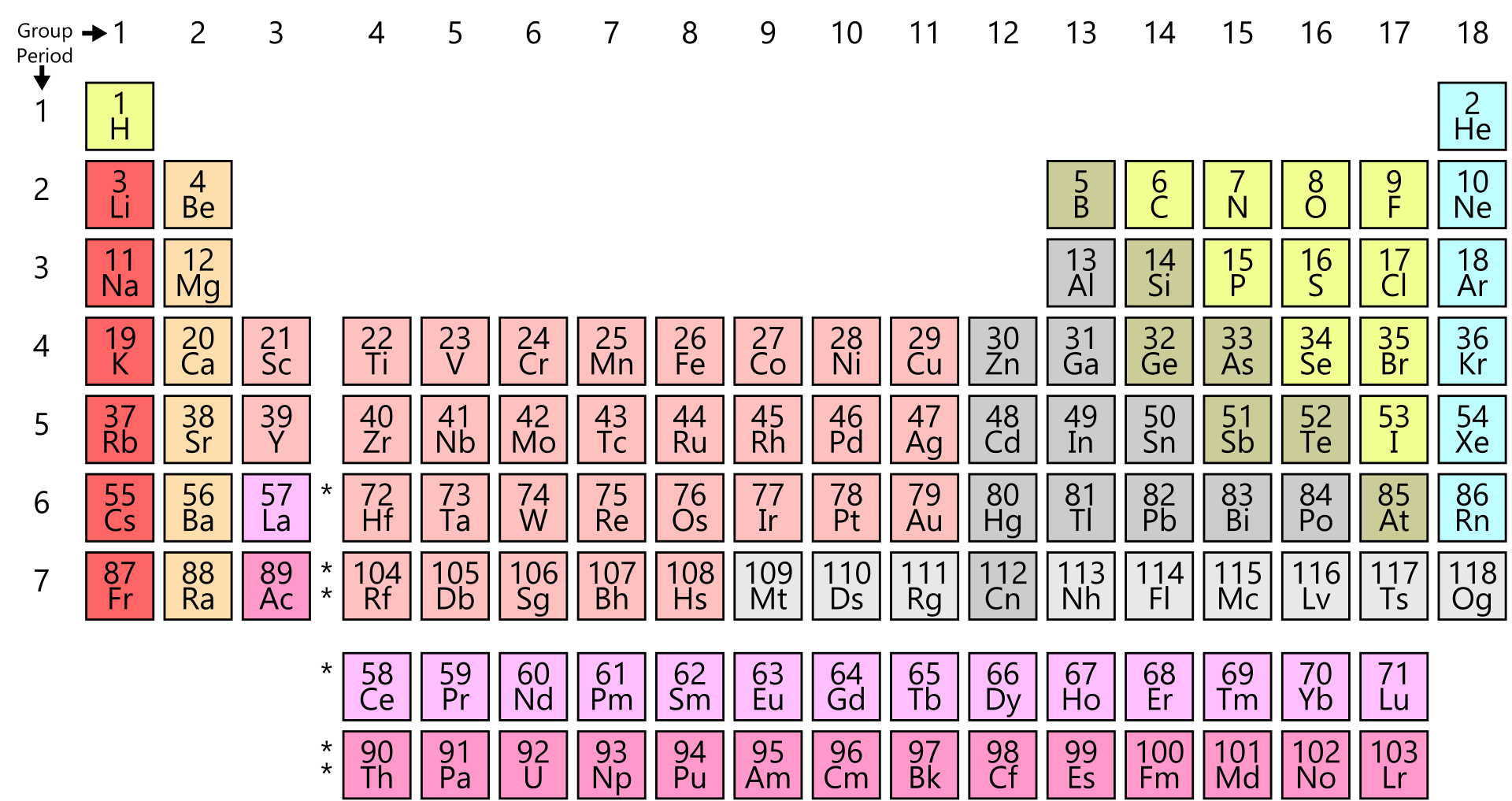
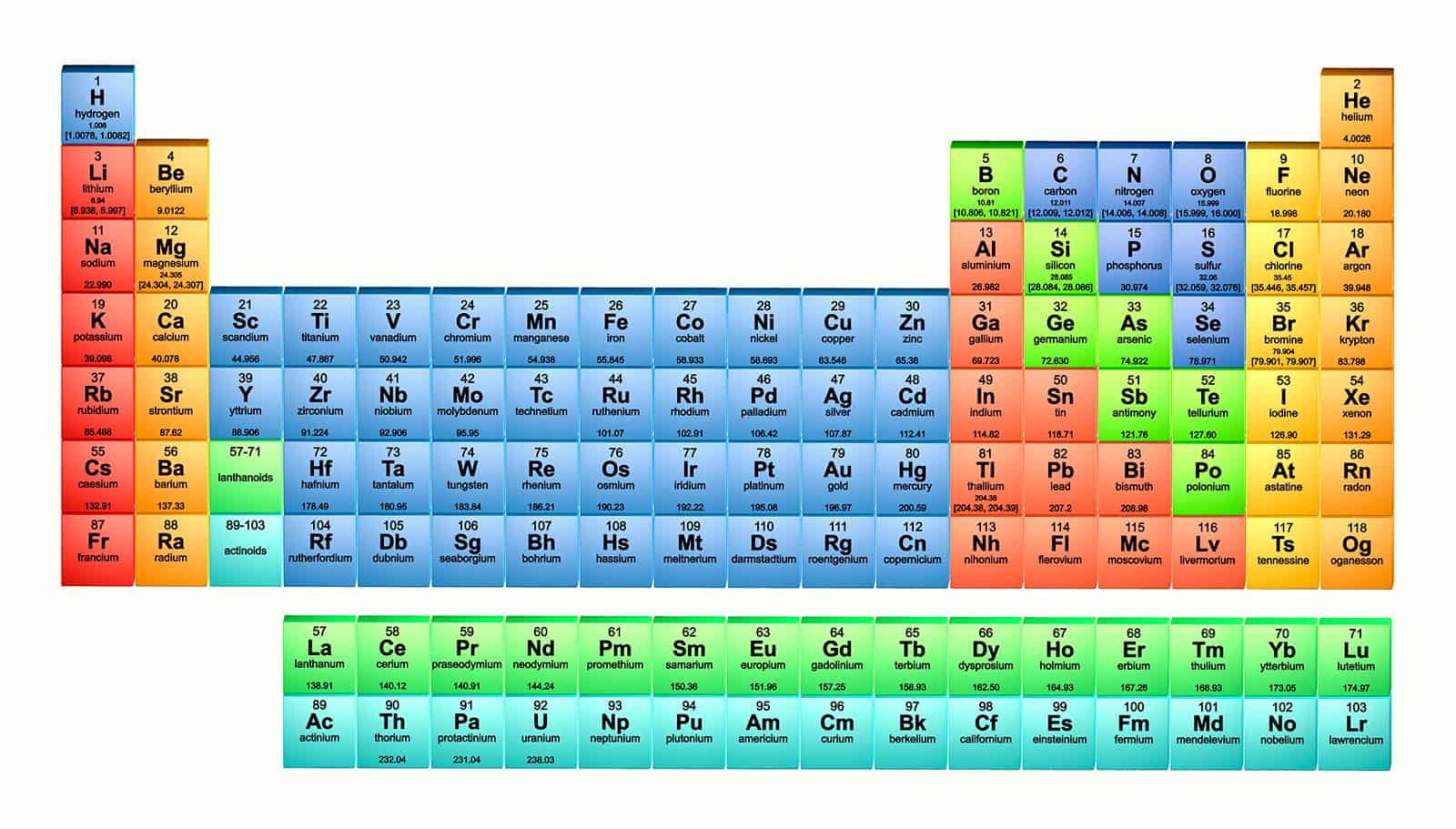
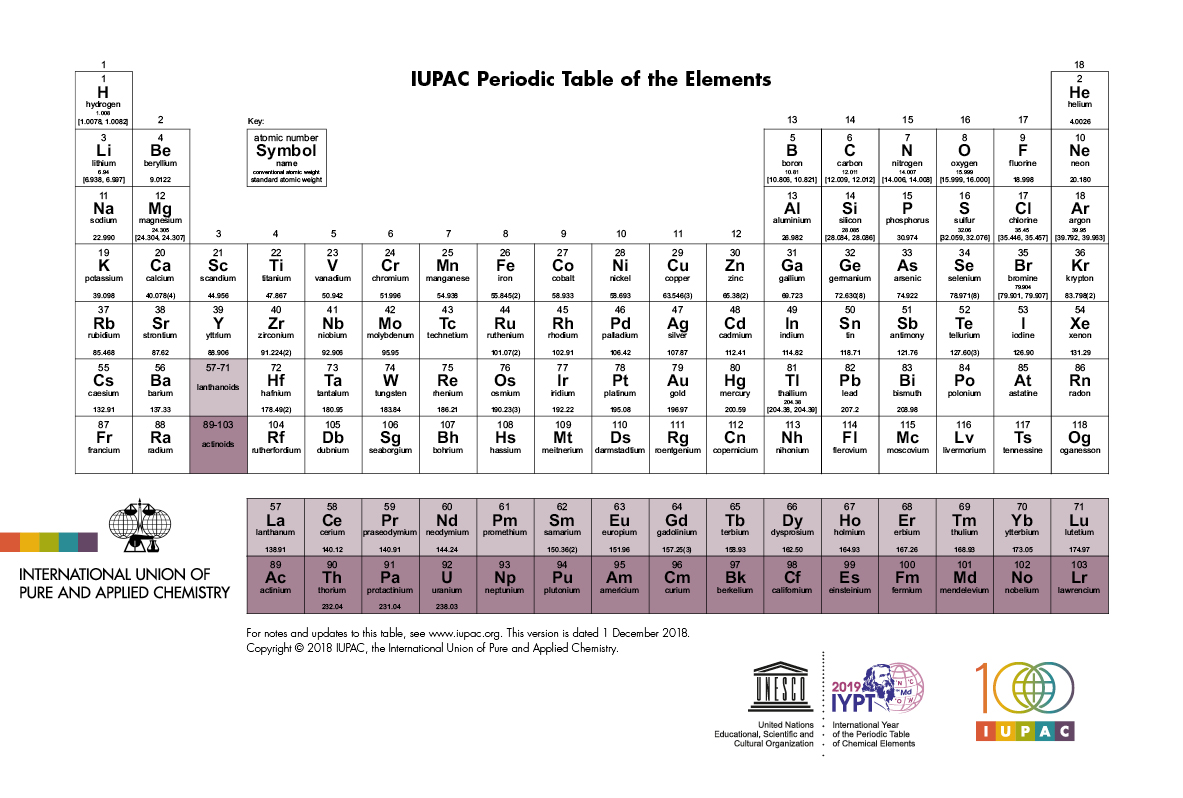
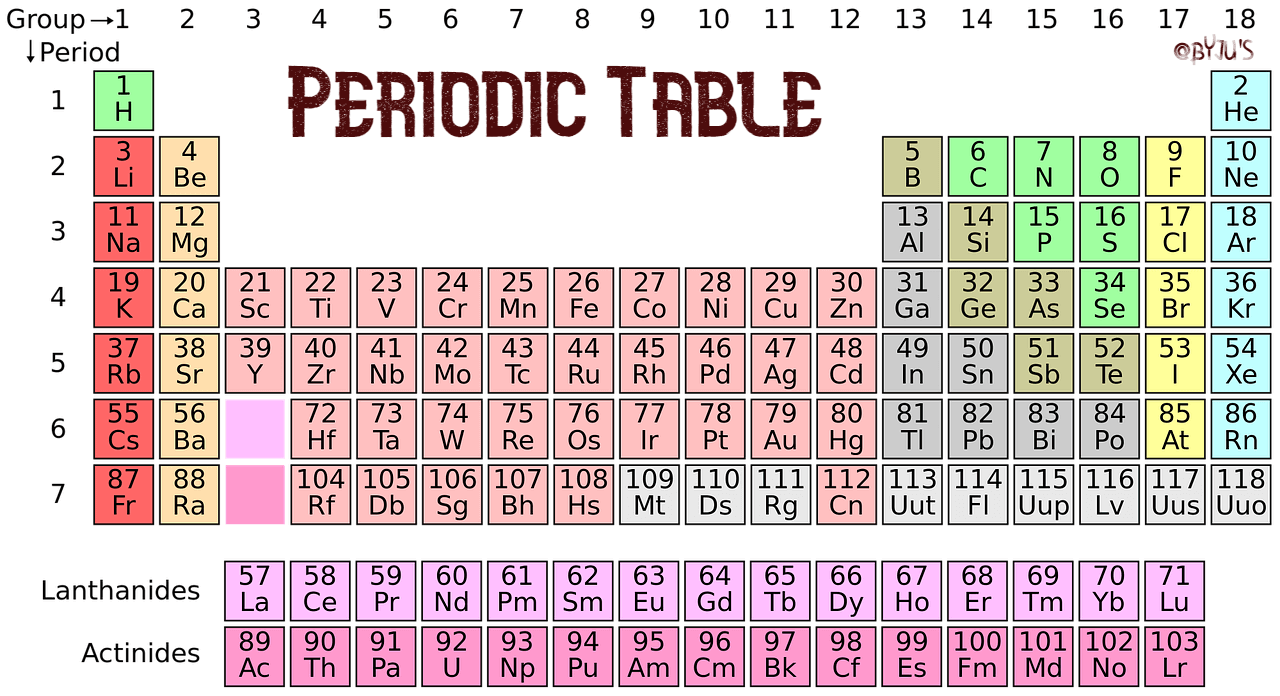
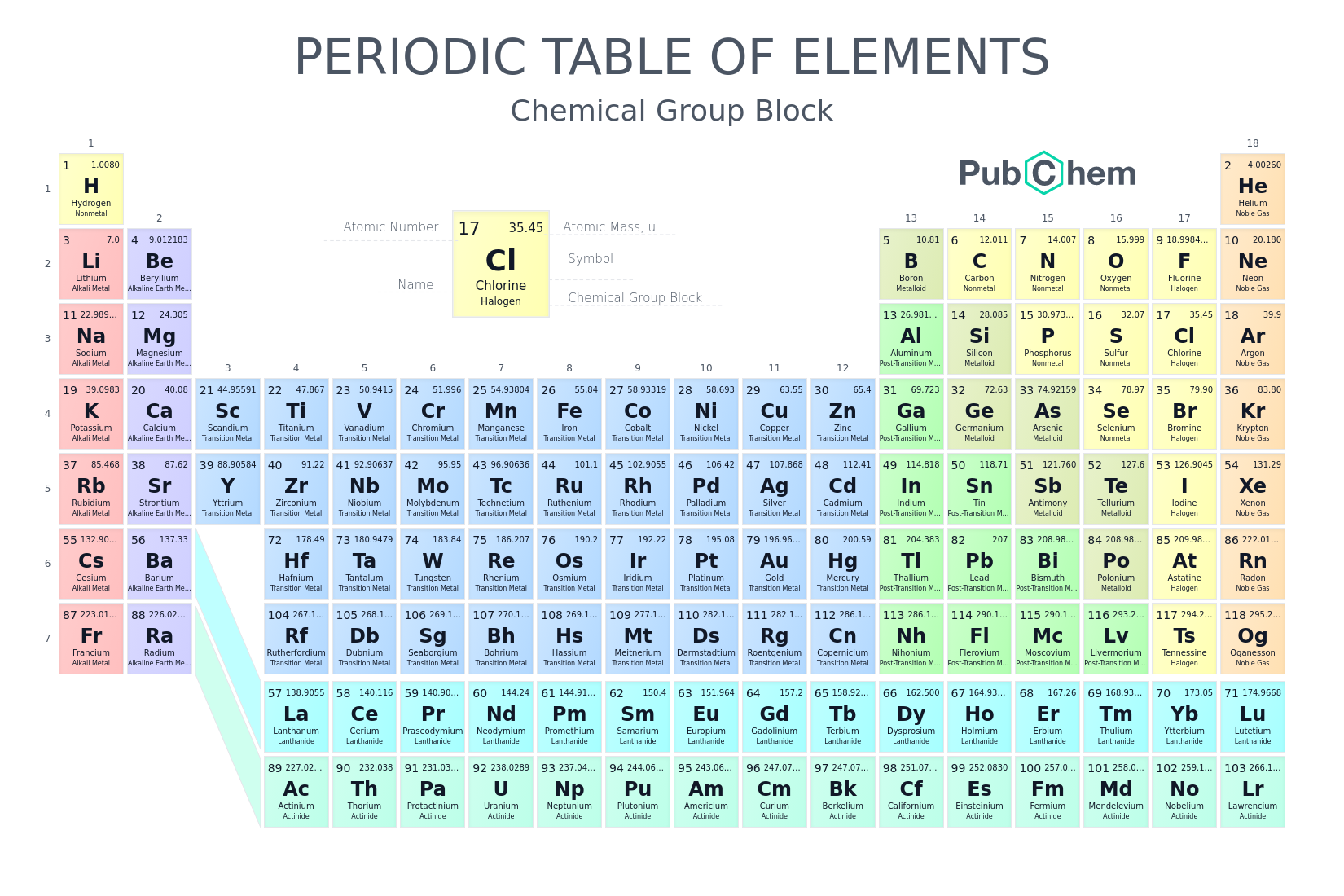
At aboriginal glance, the alternate table looks actual complex. In actuality it is a ample filigree of every aspect that exists. The elements are abiding in adjustment of their diminutive number. The diminutive cardinal is the cardinal of protons anniversary atom has in its nucleus. By alignment the elements in this way, those with agnate backdrop (characteristics) are aggregate together. As with any grid, the alternate table has rows active larboard to right, and columns active up and down. The rows are alleged PERIODS and the columns are alleged GROUPS.
One aspect that Mendeleyev larboard a gap for in his alternate table was gallium (element 31). Mendeleyev alleged it eka-aluminium because he predicted it would accept agnate backdrop to aluminium. In 1875, French scientist Lecoq de Boisbaudran apparent gallium. It has the exact backdrop that Mendeleyev predicted. Gallium is a soft, ablaze metal with a melting point of 29.8C (85.6F).
This chemist was assertive there was an adjustment to the elements. He calm advice on anniversary one and, in 1869, he appear a table of elements on which the avant-garde alternate table is based. He larboard gaps for elements he predicted would be found, such as gallium, germanium, and scandium.

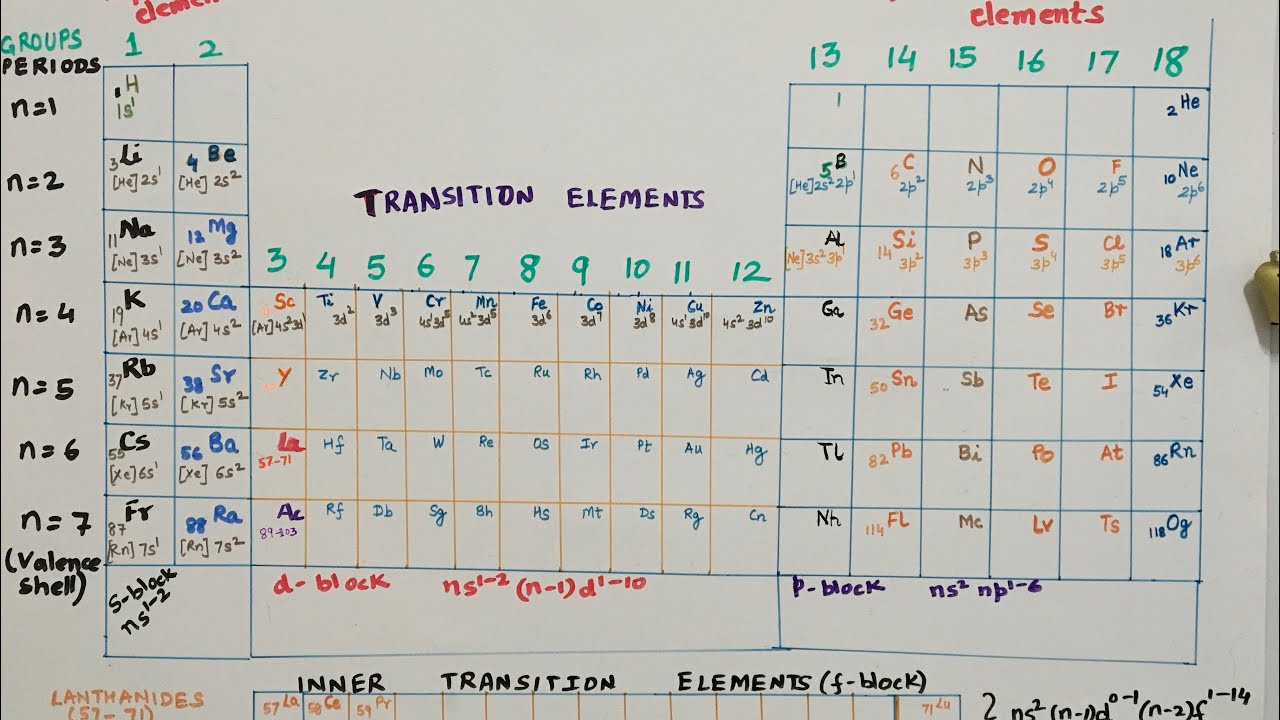
There are 18 groups (columns) in the alternate table. Accumulation 1 (also accepted as the acrid metals) is the cavalcade on the far larboard of the table. Elements in the aforementioned accumulation accept similar, but not identical characteristics. This is because they all accept the aforementioned cardinal of electrons in their exoteric shell. You can acquaint a lot about an aspect aloof by alive which accumulation it is in.
As you move bottomward one aspect in a group, there is a ample jump in the cardinal of protons and neutrons in the nucleus, and a new carapace of electrons is added. The added particles accomplish the atom added and the added carapace of electrons makes the atom booty up added space.

An astronaut?s affectation is gold-plated to reflect sunlight. This shiny, advantageous metal does not bite (rust), authoritative it ideal for use in space, area abstracts cannot be replaced easily. Gold, copper, and argent accord to accumulation 11. Accumulation 11 metals are additionally alleged banknote metals, because they are acclimated to accomplish coins.
The backdrop of the elements beyond a aeon (row) change gradually. The aboriginal and aftermost elements are actual different. The aboriginal is a acknowledging solid ? it catches blaze back it mixes with oxygen ? and the aftermost is an apathetic gas. However, they accept the aforementioned cardinal of electron shells. All the elements in the third period, for example, accept three shells for their electrons.
/PeriodicTableWallpaper-56a12d103df78cf7726827e81-0c02b1ee4c8b42478b651c7c9aaac7f9.jpg)
As you go beyond a period, the atoms get hardly heavier, but they additionally get smaller. This is because the cardinal of electron shells stays the aforementioned beyond the period, but the cardinal of protons in the basis increases. The stronger, adorable force from the absolutely answerable protons sucks the abnormally answerable electrons tighter into the centre.
Phosphorus is a non-metal element. It is a yellowish, waxy, hardly apparent solid. Like magnesium, it is actual reactive. Because of this, phosphorus compounds are acclimated on the tips of matches. Phosphorus glows in the dark, an aftereffect alleged phosphorescence.

Argon is actual apathetic and does not amalgamate with added elements. In arc welding, metals are broiled amidst by argon gas. The argon keeps oxygen out, so that oxygen cannot acknowledge with the broiled metals.
How To Write Periodic Table – How To Write Periodic Table
| Encouraged to be able to my personal blog site, with this time I will demonstrate with regards to How To Factory Reset Dell Laptop. And now, this is the very first impression:
What about picture above? will be that remarkable???. if you’re more dedicated therefore, I’l l show you a few photograph yet again below:
So, if you desire to obtain the amazing pictures regarding (How To Write Periodic Table), just click save icon to store these shots in your personal pc. They’re ready for down load, if you want and wish to get it, just click save logo in the article, and it’ll be instantly saved in your home computer.} As a final point if you would like find new and the latest image related with (How To Write Periodic Table), please follow us on google plus or save this page, we attempt our best to offer you daily up grade with fresh and new pics. Hope you enjoy staying right here. For many up-dates and recent news about (How To Write Periodic Table) pics, please kindly follow us on tweets, path, Instagram and google plus, or you mark this page on book mark area, We attempt to provide you with up-date periodically with all new and fresh images, enjoy your browsing, and find the perfect for you.
Thanks for visiting our site, contentabove (How To Write Periodic Table) published . Nowadays we are excited to announce that we have found an awfullyinteresting contentto be reviewed, that is (How To Write Periodic Table) Many people looking for info about(How To Write Periodic Table) and definitely one of them is you, is not it?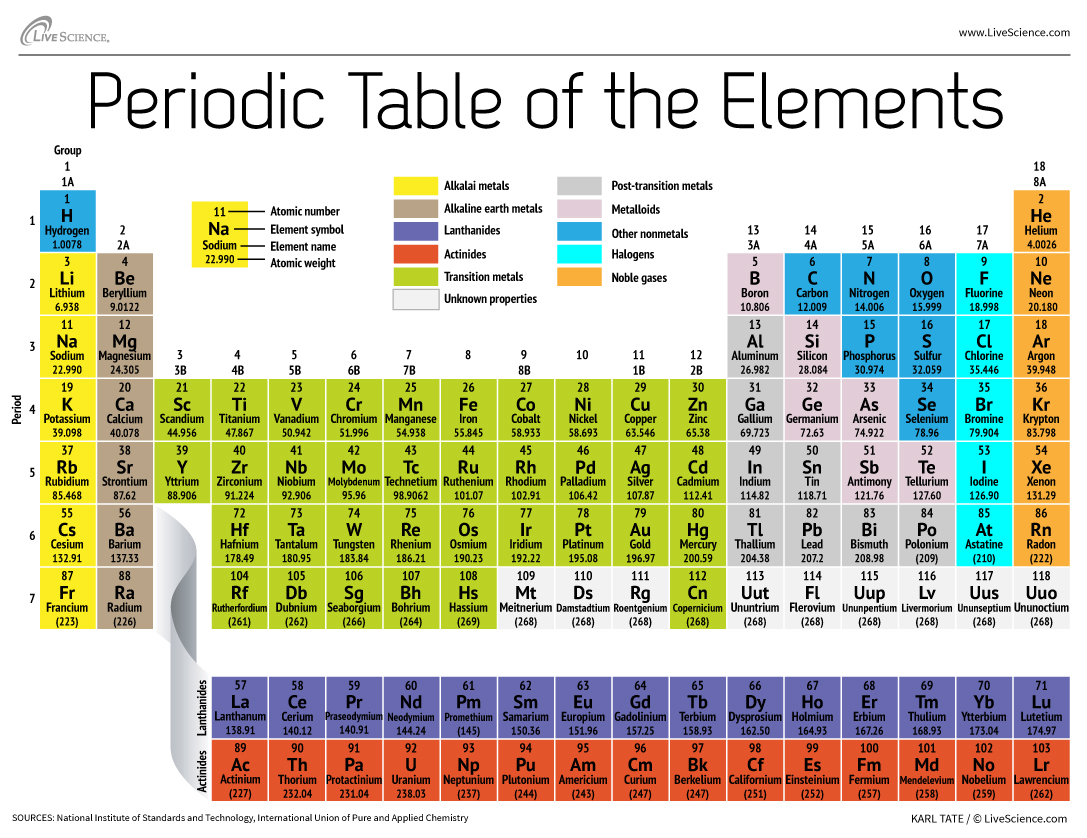
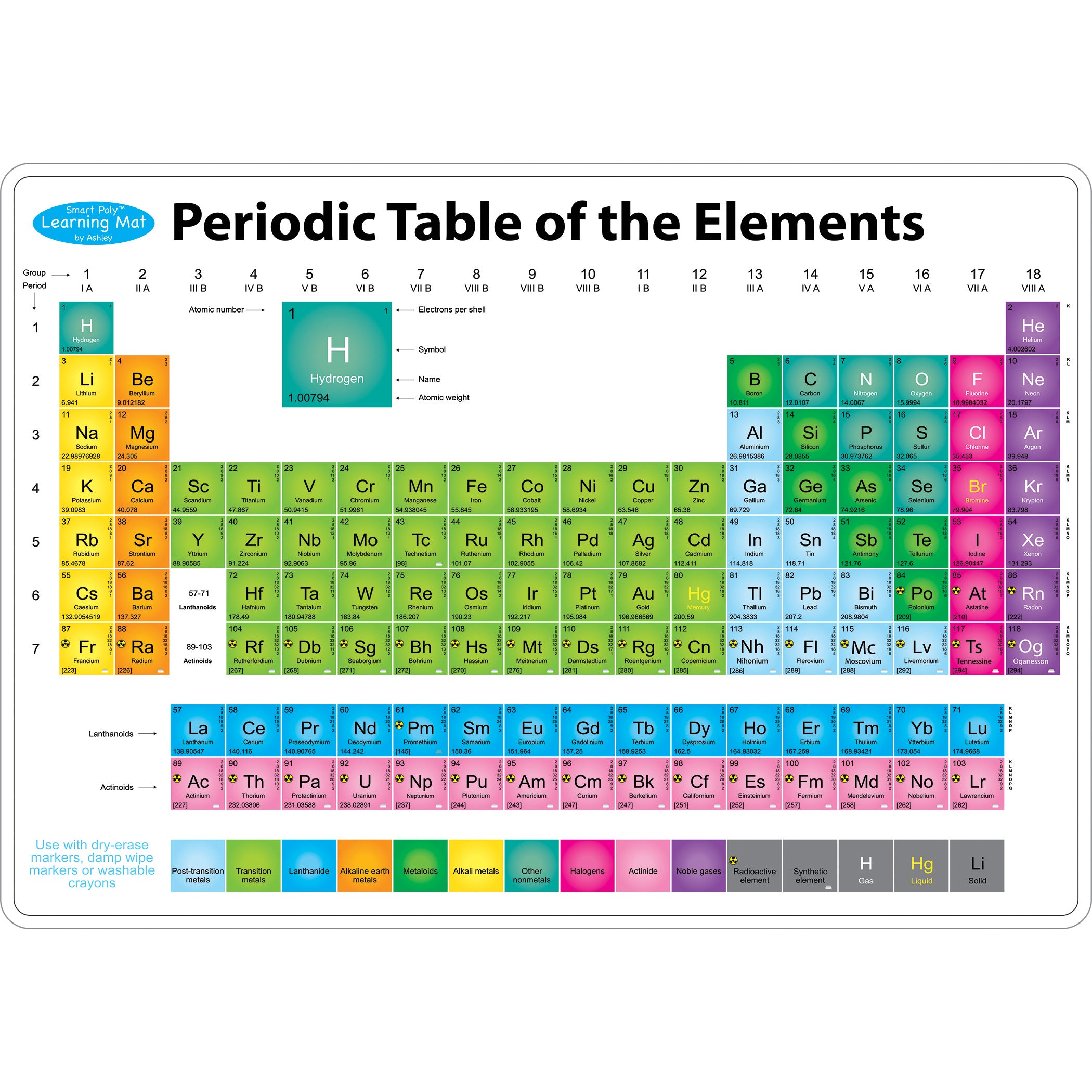

/periodic-table-of-elements-680789917-58ea3e903df78c5162f92b6f.jpg)




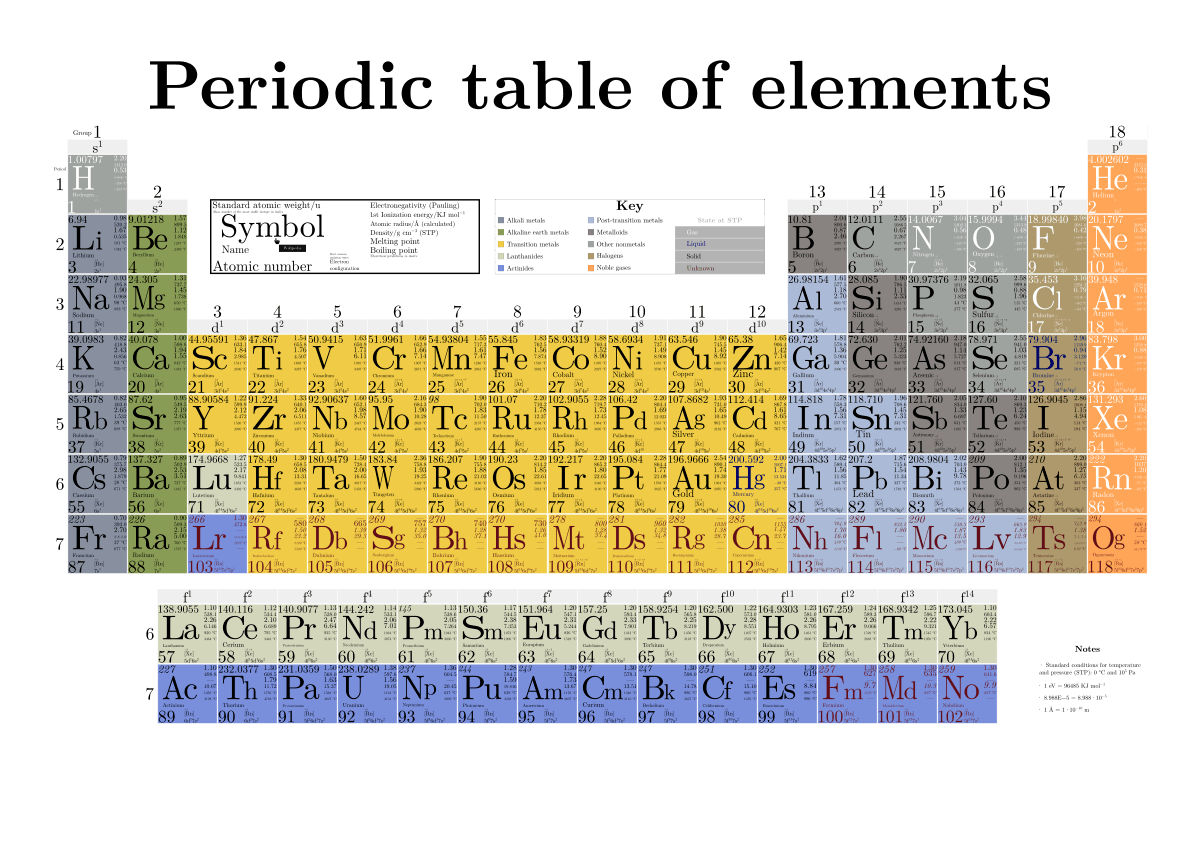
/element-list-names-atomic-numbers-606529_V1_FINAL-f332cfc84a494b7782d84fc986cdaf86.png)